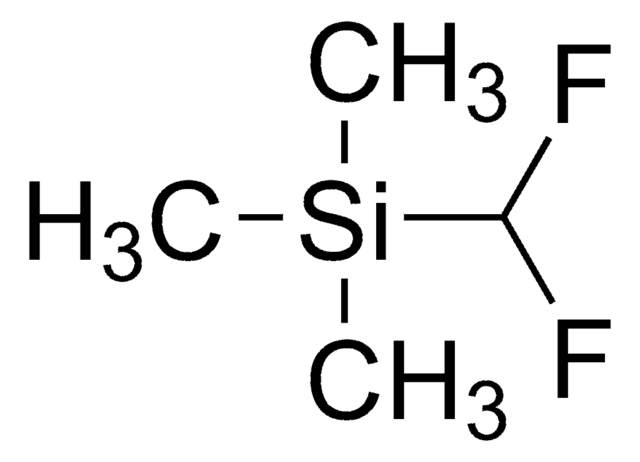
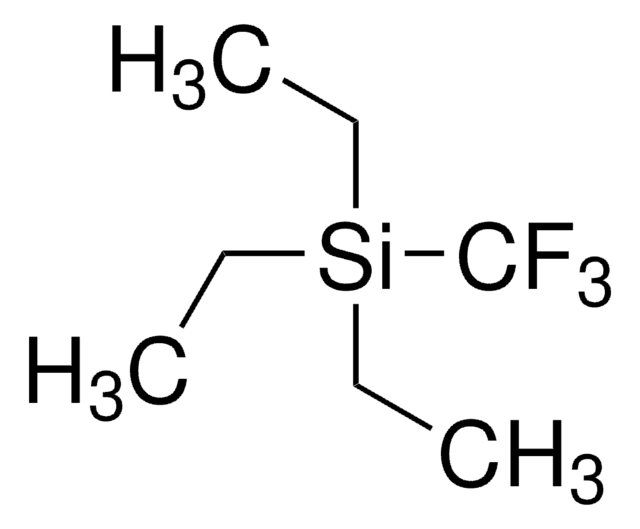
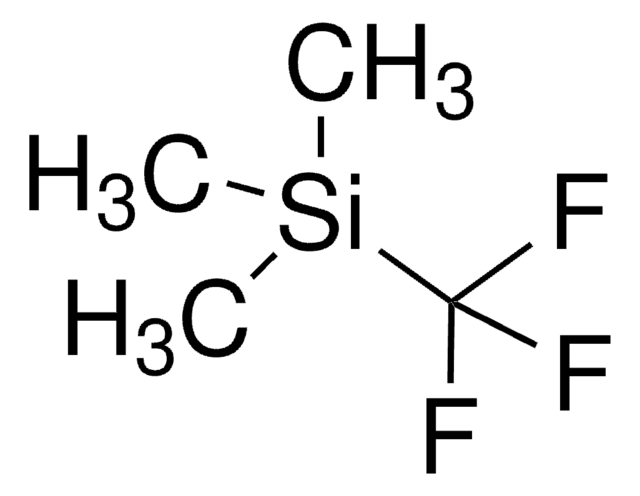
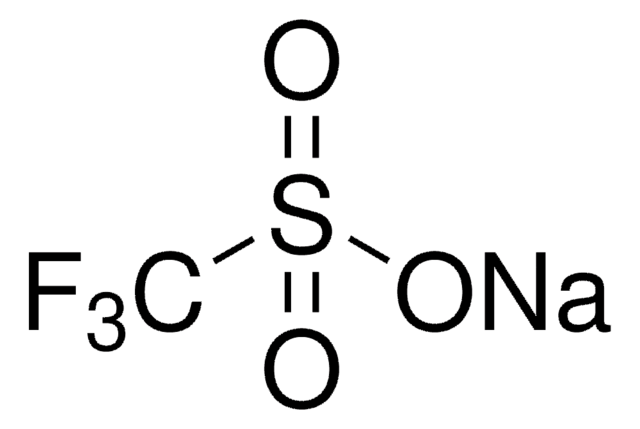
추천 제품
애플리케이션
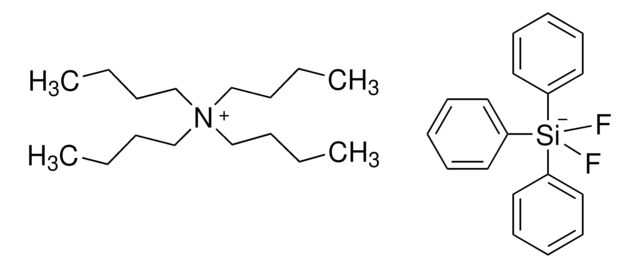
Trimethyl(trifluoromethyl)silane can be used as a trifluoromethylating agent in the following processes:
- Conversion of N-(tert-butylsulfinyl)-imines to trifluoromethylated amines
- Conversion of trans-enones to trans-α-trifluoromethyl silyl ethers
- Trifluoromethylation of azomethine imines
- Conversion of H-phosphonates to CF3-phosphonates
- Nucleophilic addition of the trifluoromethyl group to aldehydes and ketones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 2 - Water-react 2
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point (°F)
1.4 °F - closed cup
Flash Point (°C)
-17 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves
이미 열람한 고객
Craig P Johnston et al.
Journal of the American Chemical Society, 140(35), 11112-11124 (2018-08-07)
The mechanism of CF3 transfer from R3SiCF3 (R = Me, Et, iPr) to ketones and aldehydes, initiated by M+X- (<0.004 to 10 mol %), has been investigated by analysis of kinetics (variable-ratio stopped-flow NMR and IR), 13C/2H KIEs, LFER, addition
Catalytic enantioselective trifluoromethylation of azomethine imines with trimethyl (trifluoromethyl) silane
Kawai H, et al.
Angewandte Chemie (International Edition in English), 121(34), 6442-6445 (2009)
Peter T Kaplan et al.
Beilstein journal of organic chemistry, 13, 2297-2303 (2017-11-29)
A number of copper reagents were compared for their effectiveness in trifluoromethylating 4-iodobiphenyl, 4-iodotoluene, and 2-iodotoluene. Yields over time were plotted in order to refine our understanding of each reagent performance, identify any bottlenecks, and provide more insight into the
Stereoselective Nucleophilic Trifluoromethylation of N?(tert?Butylsulfinyl) imines by Using Trimethyl (trifluoromethyl) silane.
Prakash G K, et al.
Angewandte Chemie (International Edition in English), 113(3), 609-610 (2001)
CsF-catalyzed nucleophilic trifluoromethylation of trans-enones with trimethyl (trifluoromethyl) silane: A facile synthesis of trans-?-trifluoromethyl allylic alcohols.
Singh R P, et al.
Organic Letters, 1(7), 1047-1049 (1999)
문서
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.