771406
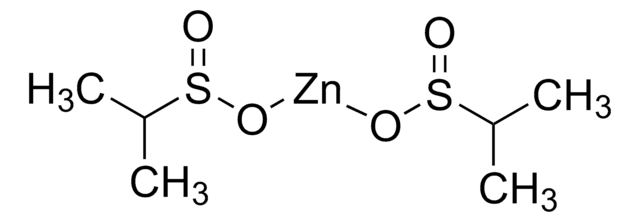
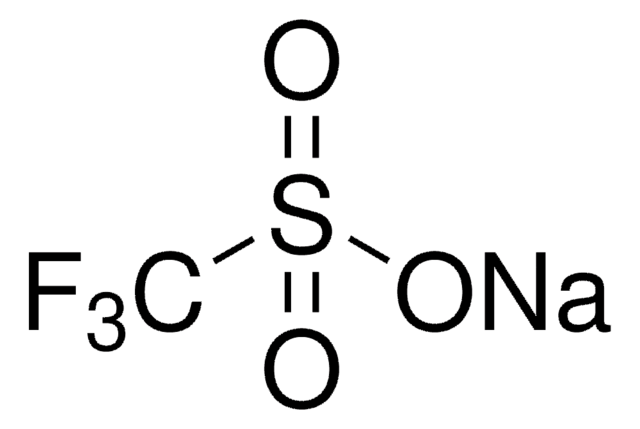
Zinc trifluoromethanesulfinate
동의어(들):
1,1,1-Trifluoro-methanesulfinic acid zinc salt (2:1), Baran trifluoromethylation reagent, Bis(((trifluoromethyl)sulfinyl)oxy)zinc, TFMS
About This Item
추천 제품
양식
solid
Quality Level
반응 적합성
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
mp
151-157 °C
저장 온도
2-8°C
SMILES string
FC(F)(F)S(=O)O[Zn]OS(=O)C(F)(F)F
InChI
1S/2CHF3O2S.Zn/c2*2-1(3,4)7(5)6;/h2*(H,5,6);/q;;+2/p-2
InChI key
UANOWFITUWBPCF-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
결합
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)