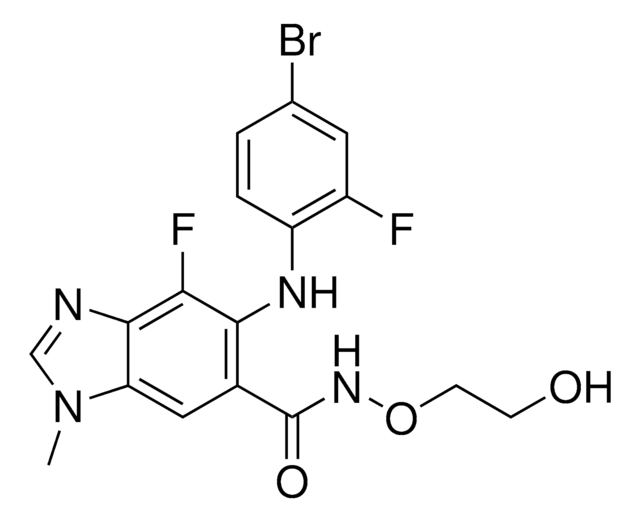
おすすめの製品
アッセイ
≥98% (HPLC)
フォーム
solid
溶解性
DMSO: 40 mg/mL
オーガナイザー
AstraZeneca
保管温度
room temp
SMILES記法
[n]2(ncnc2)Cc1cc(cc(c1)C(C)(C)C#N)C(C)(C)C#N
InChI
1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
InChI Key
YBBLVLTVTVSKRW-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
Anastrozole (aromatase inhibitor) has been used:
- as a positive control in DNA fragmentation (ladder) assay
- to investigate its effects along with extra virgin olive oil and its major fatty acid component (omega-9 OA) in estrogen receptor positive mammary adenocarcinoma cells
- to study its effects on viability, cell proliferation and apoptosis in Glioblastoma multiforme model in vivo
生物化学的/生理学的作用
アナストロゾールは非ステロイド性アロマターゼインヒビターです。
Anastrozole, which contains a triazole functional group, reversibly binds to the cytochrome P-450 component of aromatase. Binding interferes with the catalytic properties of aromatase, which results in inhibition of estrogen synthesis.
The aromatase enzyme converts adrenal androgens to estrogen; this enzymatic activity is the primary source of estrogen production in postmenopausal women. One treatment for estrogen receptor-positive breast cancer in postmenopausal women is through inhibition of aromatase. Anastrozole is a nonsteroidal, benzyl-triazole derivative that inhibits aromatase through competitive inhibition and is used to treat estrogen receptor-positive breast cancer. This compound is considered a third-generation, Type II aromatase inhibitor because it is more selective and less effective (if at all) on other steroidal hormones than first and second generation inhibitors.
特徴および利点
This compound is featured on the Nuclear Receptors (Steroids) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by AstraZeneca. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Repr. 1B
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
劇物
Jan Code
A2736-50MG:4548173298818
A2736-BULK:
A2736-10MG:4548173298801
A2736-VAR:
この製品を見ている人はこちらもチェック
Novel acylated steroidal glycosides from Caralluma tuberculata induce caspase-dependent apoptosis in cancer cells
Waheed A, et al.
Journal of Ethnopharmacology, 137(3), 1189-1196 (2011)
Yanyan Hong et al.
Steroids, 76(8), 802-806 (2011-03-23)
Aromatase is the rate-limiting enzyme in estrogen biosynthesis. As a cytochrome P450, it utilizes electrons from NADPH-cytochrome P450 reductase (CPR) to produce estrogen from androgen. Estrogen is a key factor in the promotion of hormone-dependent breast cancer growth. Aromatase inhibitors
P Dubsky et al.
Annals of oncology : official journal of the European Society for Medical Oncology, 24(3), 640-647 (2012-10-05)
In early estrogen receptor (ER)-positive/HER2-negative breast cancer, the decision to administer chemotherapy is largely based on prognostic criteria. The combined molecular/clinical EndoPredict test (EPclin) has been validated to accurately assess prognosis in this population. In this study, the clinical relevance
F Boccardo et al.
European journal of cancer (Oxford, England : 1990), 49(7), 1546-1554 (2013-02-19)
The Italian Tamoxifen Anastrozole (ITA) trial investigated the efficacy of switching to anastrozole for women who were already on adjuvant tamoxifen since 2-3years. Relapse-free survival (RFS) was the primary end-point; event-free survival (EFS), overall survival (OS) and safety were secondary
P I Petkov et al.
SAR and QSAR in environmental research, 20(7-8), 657-678 (2009-12-22)
Cytochrome P450 aromatase is a key steroidogenic enzyme that converts androgens to estrogens in vertebrates. There is much interest in aromatase inhibitors (AIs) both because of their use as pharmaceuticals in the treatment of estrogen-sensitive breast cancers, and because a
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)