おすすめの製品
フォーム
liquid
品質水準
反応適合性
reaction type: click chemistry
reagent type: linker
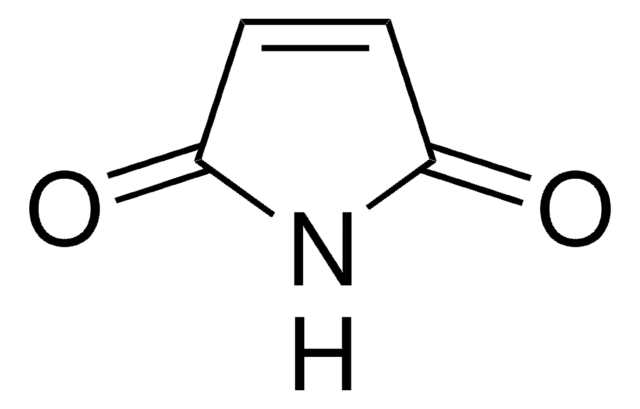
官能基
maleimide
保管温度
−20°C
SMILES記法
O=C(NCCCOCCOCCOCCCNC(CCN1C(C=CC1=O)=O)=O)OC2CCC/C=C/CC2
InChI
1S/C26H41N3O8/c30-23(12-15-29-24(31)10-11-25(29)32)27-13-6-16-34-18-20-36-21-19-35-17-7-14-28-26(33)37-22-8-4-2-1-3-5-9-22/h1-2,10-11,22H,3-9,12-21H2,(H,27,30)(H,28,33)/b2-1+
InChI Key
BJZRPWAVCDJCTC-OWOJBTEDSA-N
生物化学的/生理学的作用
Maleimide (thiol reactive) functionalized trans-cyclooctene derivative for incorporation of the cyclooctene moiety into thiol containing compounds or biomolecules. Trans-cyclooctenes are useful in strain-promoted copper-free click chemistry cycloaddition reactions with 1; 2; 4; 5-tetrazines. This cyclooctene will react with tetrazine functionalized compounds or biomolecules without the need for a catalyst to result in a stable covalent linkage. The 4+2 inverse electron demand Diels-Alder cycloaddition between trans-cyclooctene and tetrazines is the fastest biologically compatible ligation technology reported and has had many applications in biological labeling and imaging. The PEG spacer allows for increased water solubility; less aggregation and an increased distance between the thiol to be modified and the reactive alkene.
関連製品
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
René Platzer et al.
Nature communications, 11(1), 4993-4993 (2020-10-07)
Determining nanoscale protein distribution via Photoactivated Localization Microscopy (PALM) mandates precise knowledge of the applied fluorophore's blinking properties to counteract overcounting artifacts that distort the resulting biomolecular distributions. Here, we present a readily applicable methodology to determine, optimize and quantitatively
Mark R Karver et al.
Bioconjugate chemistry, 22(11), 2263-2270 (2011-09-29)
1,2,4,5-Tetrazines have been established as effective dienes for inverse electron demand [4 + 2] Diels-Alder cycloaddition reactions with strained alkenes for over 50 years. Recently, this reaction pair combination has been applied to bioorthogonal labeling and cell detection applications; however
Neal K Devaraj et al.
Bioconjugate chemistry, 19(12), 2297-2299 (2008-12-05)
Bioorthogonal tetrazine cycloadditions have been applied to live cell labeling. Tetrazines react irreversibly with the strained dienophile norbornene forming dihydropyrazine products and dinitrogen. The reaction is high yielding, selective, and fast in aqueous media. Her2/neu receptors on live human breast
Melissa L Blackman et al.
Journal of the American Chemical Society, 130(41), 13518-13519 (2008-09-19)
Described is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality and proceed in high yield in organic solvents, water
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)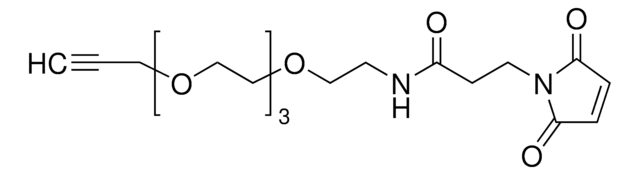

![9-Oxabicyclo[6.1.0]non-4-ene 95%](/deepweb/assets/sigmaaldrich/product/structures/328/338/c44d0a8d-81ab-4a17-81bb-aebb26a006e7/640/c44d0a8d-81ab-4a17-81bb-aebb26a006e7.png)

![(1R,8S,9s)-ビシクロ[6.1.0]ノン-4-イン-9-イルメチルN-スクシンイミジルカーボナート for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)