おすすめの製品
フォーム
solid
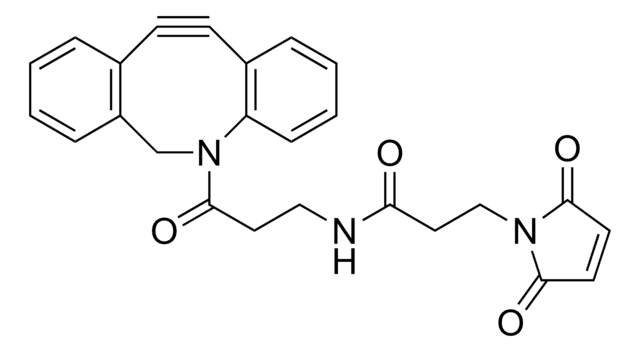
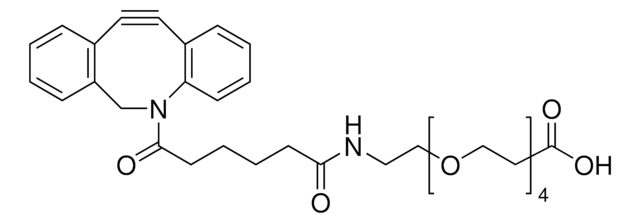
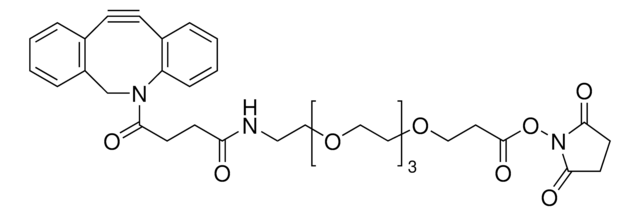
反応適合性
reaction type: click chemistry
reagent type: cross-linking reagent
保管温度
−20°C
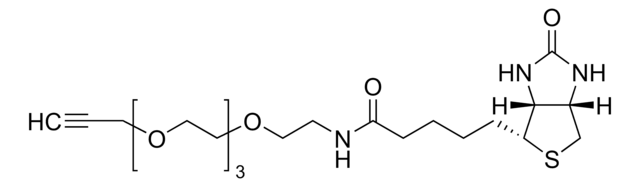
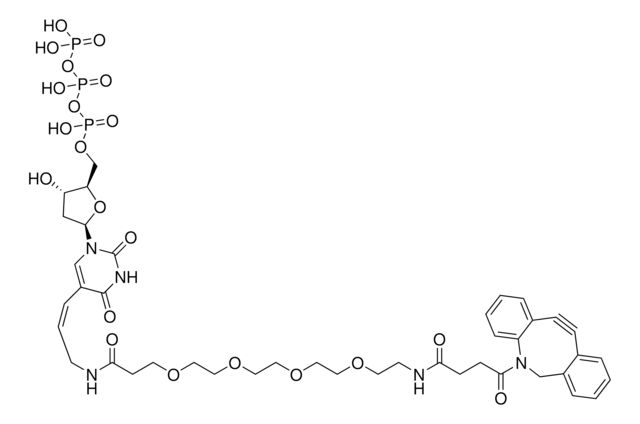
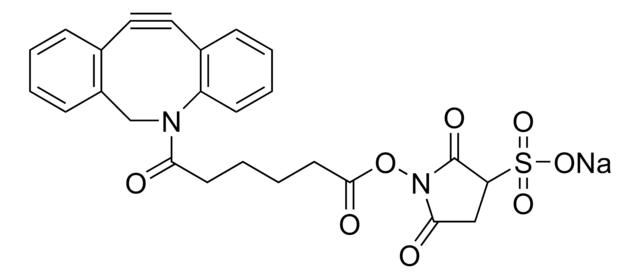
SMILES記法
CC[NH+](CC)CC.[O-]S(=O)(=O)C(CNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)C(=O)NCCC(=O)N3Cc4ccccc4C#Cc5ccccc35
InChI
1S/C31H35N5O7S2.C6H15N/c37-27(12-6-5-11-25-29-23(19-44-25)34-31(40)35-29)33-17-26(45(41,42)43)30(39)32-16-15-28(38)36-18-22-9-2-1-7-20(22)13-14-21-8-3-4-10-24(21)36;1-4-7(5-2)6-3/h1-4,7-10,23,25-26,29H,5-6,11-12,15-19H2,(H,32,39)(H,33,37)(H2,34,35,40)(H,41,42,43);4-6H2,1-3H3/t23-,25-,26?,29-;/m0./s1
InChI Key
RACLEVCKGUBCTH-GESOYTMLSA-N
詳細
アプリケーション
特徴および利点
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
760706-BULK:
760706-5MG:
760706-VAR:
この製品を見ている人はこちらもチェック
資料
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)
![N-[(1R,8S,9s)-ビシクロ[6.1.0]ノン-4-イン-9-イル メトキシカルボニル]-1,8-ジアミノ-3,6-ジオキサオクタン for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)