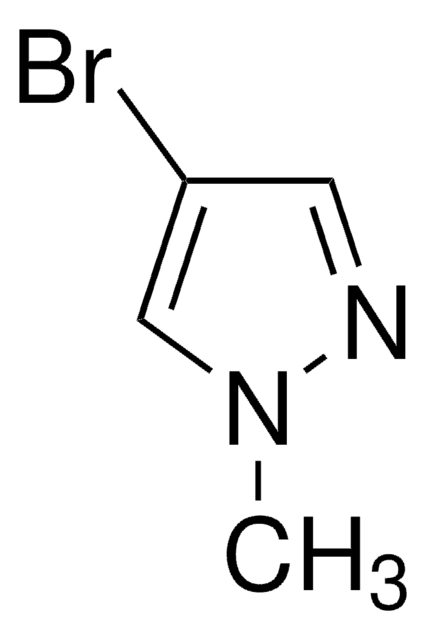
おすすめの製品
品質水準
アッセイ
95%
フォーム
solid
mp
59-64 °C (lit.)
SMILES記法
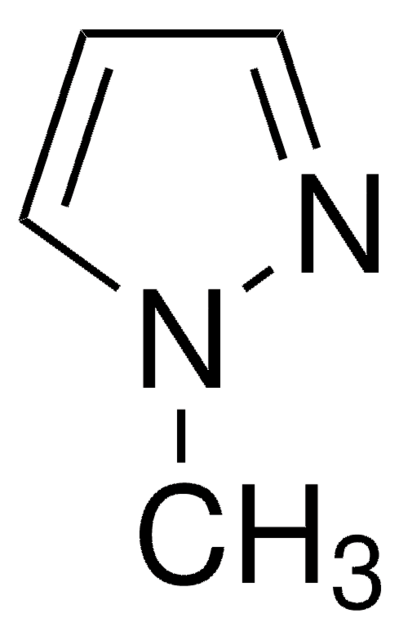
Cn1cc(cn1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C10H17BN2O2/c1-9(2)10(3,4)15-11(14-9)8-6-12-13(5)7-8/h6-7H,1-5H3
InChI Key
UCNGGGYMLHAMJG-UHFFFAOYSA-N
アプリケーション
Reagent used for
Reagent used for preparation of
- Suzuki-Miyaura cross-coupling reactions
- Transesterification reactions
Reagent used for preparation of
- Aminothiazoles as γ-secretase modulators
- Amino-pyrido-indol-carboxamides, as potential JAK2 inhibitors for myeloproliferative disorders therapy
- Pyridine derivatives as TGF-β1 and activin A signalling inhibitors
- MK-2461 analogs as inhibitors of c-Met kinase for the treatment of cancer
Stable alternative to the boronic acid for Suzuki-Miyaura palladium-catalyzed cross-coupling
保管分類コード
11 - Combustible Solids
WGK
WGK 3
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
PRTR
第一種指定化学物質
Jan Code
595314-VAR:
595314-25G:
595314-5G:
595314-1G:
595314-BULK:
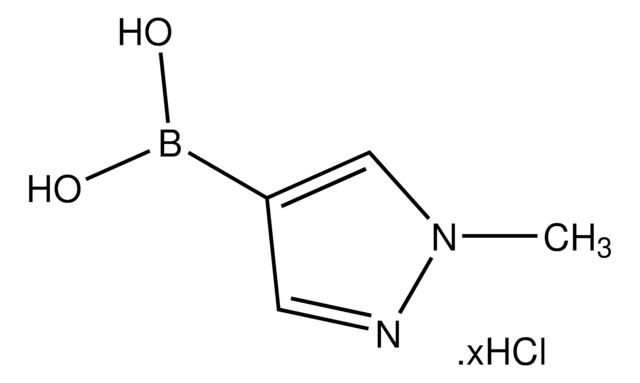
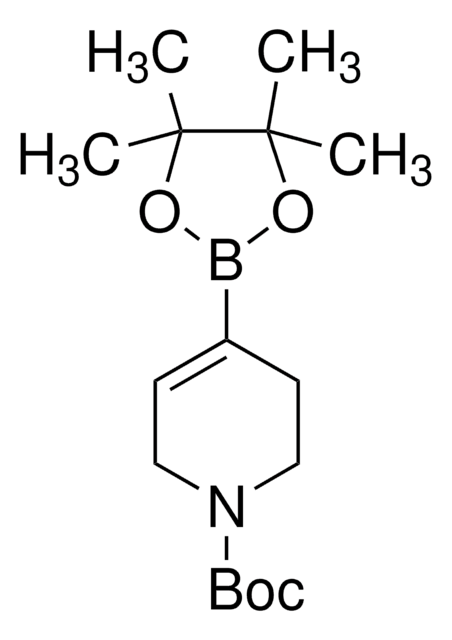
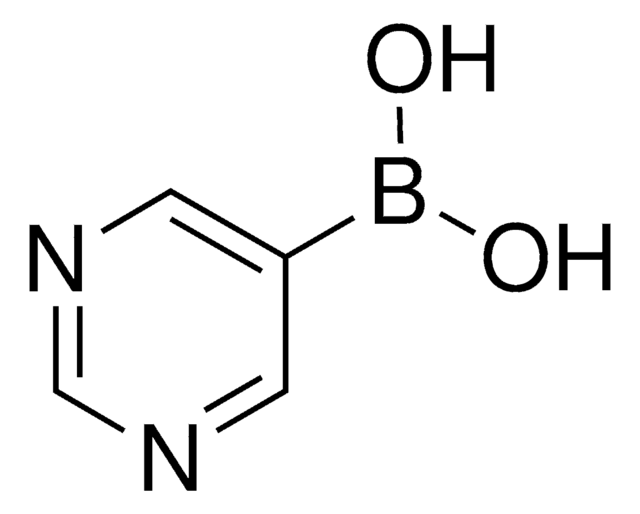
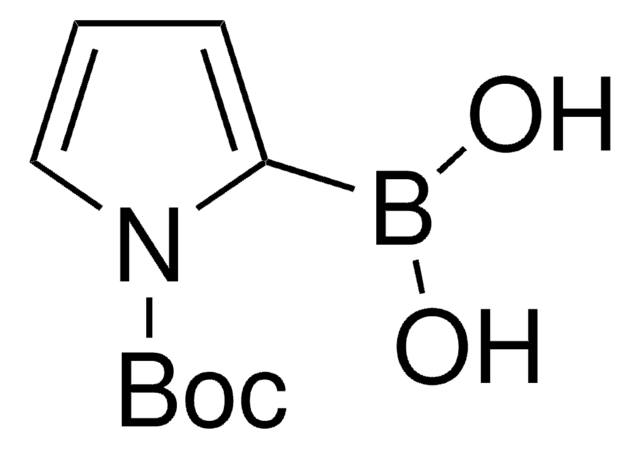
この製品を見ている人はこちらもチェック
Michael Lainchbury et al.
Journal of medicinal chemistry, 55(22), 10229-10240 (2012-10-23)
Inhibitors of checkpoint kinase 1 (CHK1) are of current interest as potential antitumor agents, but the most advanced inhibitor series reported to date are not orally bioavailable. A novel series of potent and orally bioavailable 3-alkoxyamino-5-(pyridin-2-ylamino)pyrazine-2-carbonitrile CHK1 inhibitors was generated
Rudy Ciayadi et al.
Bioorganic & medicinal chemistry letters, 21(18), 5642-5645 (2011-07-26)
Novel inhibitors of TGF-β1 and activin A signalling based on a 2-aryl-4-(3-(pyridin-2-yl)-1H-pyrazol-4-yl)pyridine pharmacophore have been synthesised. Compounds containing phenyl or aromatic nitrogen heterocycle substituents inhibited both types of signalling with HEK-293T cells in culture, with a selectivity preference for TGF-β1.
Direct conversion of pinacol arylboronic esters to aryl triolborates
Li, G.-Q.; et al.
Chemistry Letters (Jpn), 40, 702-704 (2011)
Brijesh Bhayana et al.
Organic letters, 11(17), 3954-3957 (2009-08-12)
A catalyst system for the Suzuki-Miyaura cross-coupling reactions of aryl and vinyl tosylates and mesylates has been developed. This catalyst displays excellent functional group tolerance and allows the coupling of heteroarylboronic acids with aryl tosylates and mesylates to be performed
Thomas Lübbers et al.
Bioorganic & medicinal chemistry letters, 21(21), 6554-6558 (2011-09-20)
We herein report the discovery of a new γ-secretase modulator class with an aminothiazole core starting from a HTS hit (3). Synthesis and SAR of this series are discussed. These novel compounds demonstrate moderate to good in vitro potency in
資料
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Global Trade Item Number
| カタログ番号 | GTIN |
|---|---|
| 595314-25G | 4061833399224 |
| 595314-1G | 4061832644059 |
| 595314-5G | 4061833499467 |
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)
![[1,1′-ビス(ジフェニルホスフィノ)フェロセン]ジクロロパラジウム(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![1-[1-(2-Methylphenyl)-1H-pyrazol-4-yl]methanamine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/193/071/8c363ad6-8306-4c4d-b322-749ff2feff6f/640/8c363ad6-8306-4c4d-b322-749ff2feff6f.png)
![4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrrolo[2,3-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/272/176/ea333f93-763d-458c-a328-3969b7d46e5d/640/ea333f93-763d-458c-a328-3969b7d46e5d.png)