G0639
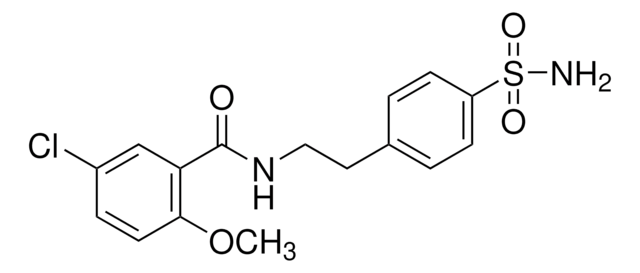
Glibenclamide
≥99% (HPLC)
Sinonimo/i:
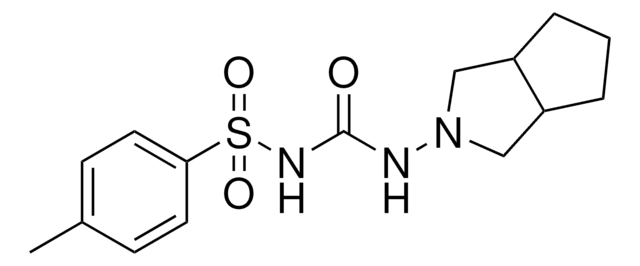
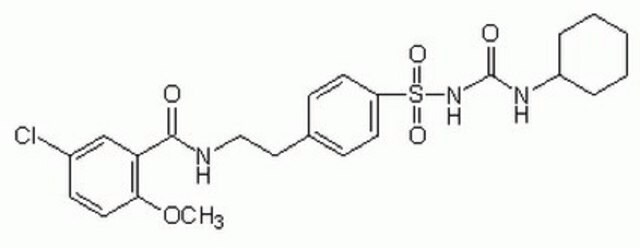
5-cloro-N-[4-(cicloesilureidosolfonil)fenetil]-2-metossibenzamide, Gliburide, N-p-[2-(5-cloro-2-metossibenzamido)etil]benzenesolfonil-N′-cicloesilurea
About This Item
Prodotti consigliati
Saggio
≥99% (HPLC)
Solubilità
ethanol: 2 mg/mL
DMSO: soluble
H2O: insoluble
Ideatore
Roche
Temperatura di conservazione
2-8°C
Stringa SMILE
COc1ccc(Cl)cc1C(=O)NCCc2ccc(cc2)S(=O)(=O)NC(=O)NC3CCCCC3
InChI
1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
ZNNLBTZKUZBEKO-UHFFFAOYSA-N
Informazioni sul gene
human ... ABCC8(6833) , KCNH2(3757) , KCNJ1(3758) , KCNJ11(3767)
rat ... Kcnj1(24521)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- as a positive control oral hypoglycemic drug to study the hypoglycemic effects of Chlorella in streptozotocin-induced diabetic mice
- as a K+ATP channel antagonist in canine with induced acute hypoxia
- as an inhibitor of cystic fibrosis transmembrane conductance regulator (CFTR) channel in fetal distal lung epithelial (FDLE) cells
Azioni biochim/fisiol
Caratteristiche e vantaggi
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 4
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.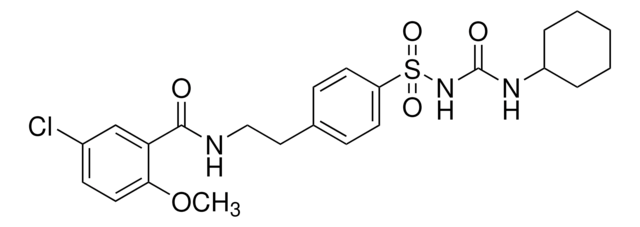





![1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one powder](/deepweb/assets/sigmaaldrich/product/structures/764/715/605dc5a5-0864-471b-a71e-bd2aa6553c1d/640/605dc5a5-0864-471b-a71e-bd2aa6553c1d.png)