S6196
Simvastatin
≥97% (HPLC), solid
Sinonimo/i:
MK-733, SVA
About This Item
Prodotti consigliati
Saggio
≥97% (HPLC)
Stato
solid
Colore
white
Punto di fusione
127-132 °C (lit.)
Solubilità
DMSO: ≥20 mg/mL
Ideatore
Merck & Co., Inc., Kenilworth, NJ, U.S.
Temperatura di conservazione
2-8°C
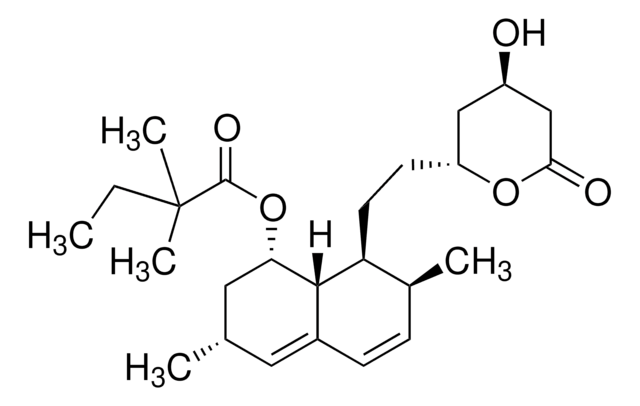
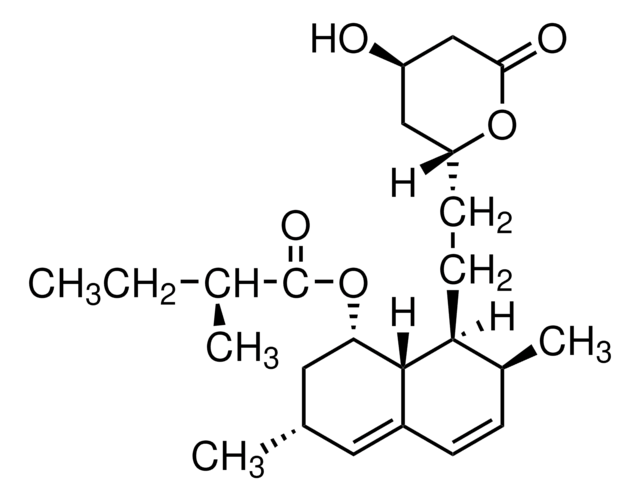
Stringa SMILE
[H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]3C[C@@H](O)CC(=O)O3)OC(=O)C(C)(C)CC
InChI
1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1
RYMZZMVNJRMUDD-HGQWONQESA-N
Informazioni sul gene
human ... HMGCR(3156)
rat ... Hmgcr(25675)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as an inhibitor of HMG CoA reductase (HMGCR)
- to study its effects on epithelial to mesenchymal transition (EMT) and the prognosis of patients with lung adenocarcinoma
- in in vivo studies to test its effect on brain tumor−initiating cells (BTIC) viability and cell proliferation
- to study the role of adenosine triphosphate (ATP)-binding cassette transporter A7 in phagocytosis of Jurkat cells
- to study the effect on endothelial dysfunction and inflammation in mice
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.