G2167
Gliclazide
powder, ≥98%
Sinonimo/i:
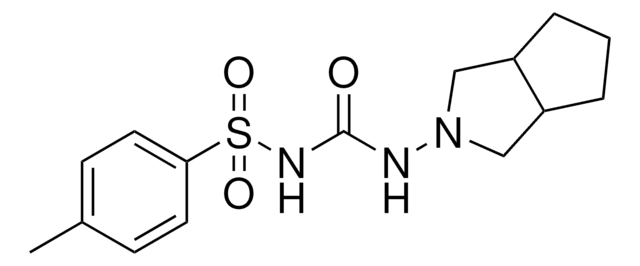
1-(3-Azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥98%
Stato
powder
Colore
white
Punto di fusione
163-169 °C (lit.)
Solubilità
methylene chloride: soluble
Ideatore
Servier
Stringa SMILE
Cc1ccc(cc1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
InChI
1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
BOVGTQGAOIONJV-UHFFFAOYSA-N
Informazioni sul gene
human ... KCNJ1(3758)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- to study its antiglycative properties and to study its effects on the glycation of bovine serum albumin (BSA) structure
- as a reference standard in solid-phase extraction (SPE) followed by instrumental analysis using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) for the quantification of water samples
- to develop and validate a simple, sensitive, rapid, economic and isocratic high-performance liquid chromatography (HPLC) method for the determination of gliclazide
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.