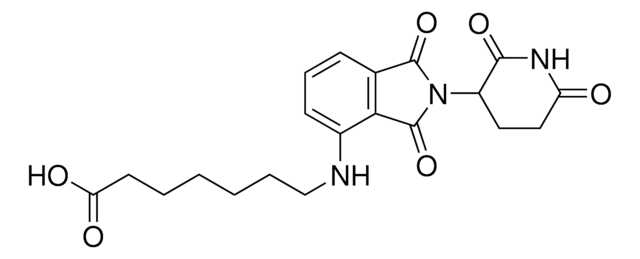
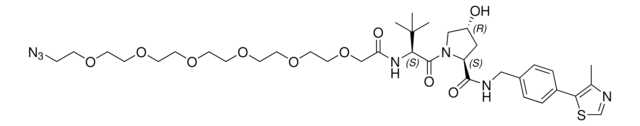
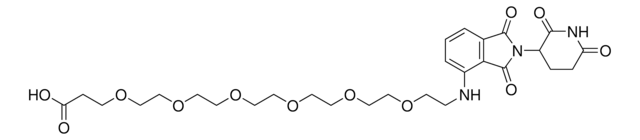
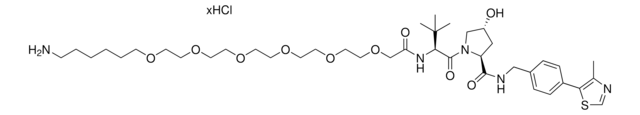
901850
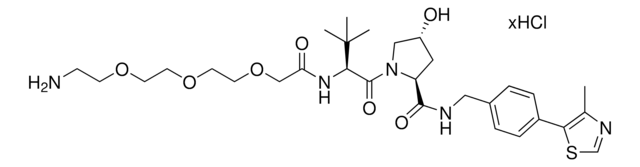
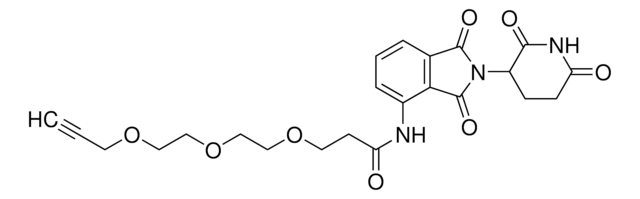
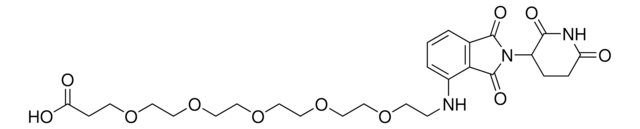
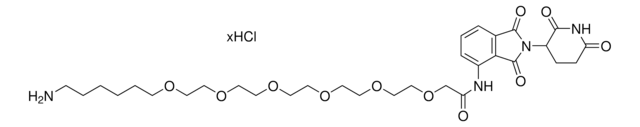
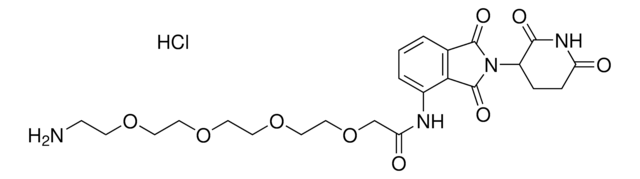
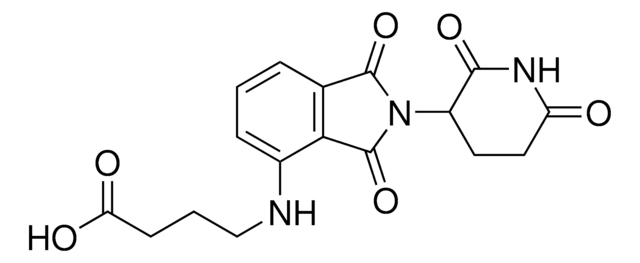
(S,R,S)-AHPC-PEG5-NH2 hydrochloride
Sinónimos:
(2S,4R)-1-((S)-20-Amino-2-(tert-butyl)-4-oxo-6,9,12,15,18-pentaoxa-3-azaicosanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride, Crosslinker−E3 ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader, VH032 conjugate
About This Item
Productos recomendados
formulario
crystals
idoneidad de la reacción
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
O=C(COCCOCCOCCOCCOCCN)N[C@H](C(N1[C@H](C(NCC2=CC=C(C3=C(C)N=CS3)C=C2)=O)C[C@@H](O)C1)=O)C(C)(C)C.Cl
Categorías relacionadas
Aplicación
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Otras notas
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Información legal
Producto relacionado
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico