T9698
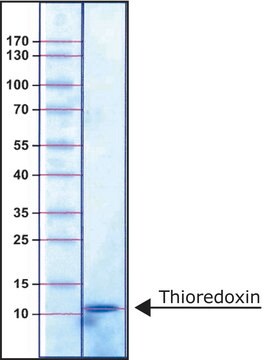
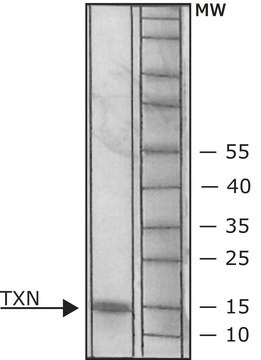
Thioredoxin Reductase from rat liver
buffered aqueous glycerol solution, ≥100 units/mg protein (Bradford)
Sinonimo/i:
NADPH:Oxidised Thioredoxin Oxidoreductase, Thioredoxin: NADP+ Oxidoreductase
About This Item
Prodotti consigliati
Origine biologica
rat liver
Livello qualitativo
Saggio
≥90% (GE)
Forma fisica
buffered aqueous glycerol solution
Attività specifica
≥100 units/mg protein (Bradford)
PM
55—67 kDa
tecniche
activity assay: suitable
Impurezze
Glutathione reductase
Solubilità
water: soluble
Compatibilità
suitable for molecular biology
N° accesso UniProt
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
Informazioni sul gene
rat ... Txnrd1(58819)
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Definizione di unità
Stato fisico
Codice della classe di stoccaggio
12 - Non Combustible Liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Oxidative stress is mediated, in part, by reactive oxygen species produced by multiple cellular processes and controlled by cellular antioxidant mechanisms such as enzymatic scavengers or antioxidant modulators. Free radicals, such as reactive oxygen species, cause cellular damage via cellular.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.