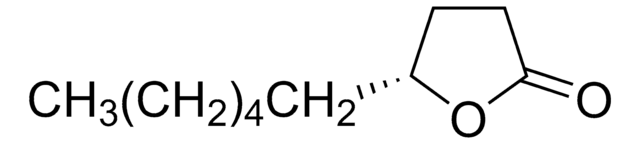W253901
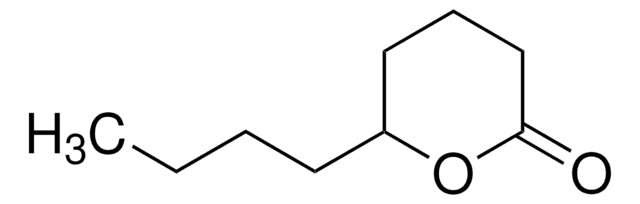
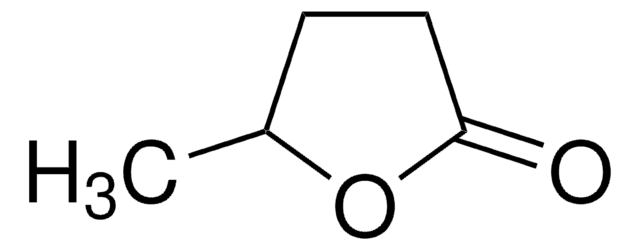
γ-Heptalactone
≥98%, FCC, FG
Sinonimo/i:
(±)-4-Heptanolide, (±)-γ-Propyl-γ-butyrolactone, (±)-Dihydro-5-propyl-2(3H)-furanone
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Grado
FG
Fragrance grade
Halal
Kosher
agenzia
follows IFRA guidelines
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
Saggio
≥98%
Indice di rifrazione
n20/D 1.442 (lit.)
P. ebollizione
61-62 °C/2 mmHg (lit.)
Densità
0.999 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Organolettico
coconut; creamy; sweet
Stringa SMILE
CCCC1CCC(=O)O1
InChI
1S/C7H12O2/c1-2-3-6-4-5-7(8)9-6/h6H,2-5H2,1H3
VLSVVMPLPMNWBH-UHFFFAOYSA-N
Informazioni sul gene
human ... CYP1A2(1544)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Enzymes with Lactonase Activity against Fungal Quorum Molecules as Effective Antifungals.: This study investigates the potential of lactonase enzymes in combating fungal infections by disrupting fungal communication systems. The findings indicate significant antifungal activity, suggesting applications in developing new antifungal treatments (Efremenko et al., 2024).
- Product study of the reactions of gamma-caprolactone and gamma-heptalactone initiated by OH radicals at 298 K and atmospheric pressure: Formation of acyl peroxynitrates (APN).: This research explores the chemical reactions of gamma-heptalactone with OH radicals, leading to the formation of acyl peroxynitrates, which are significant in atmospheric chemistry and pollution studies (Baptista et al., 2023).
- Effect of gamma-Heptalactone on the Morphology and Production of Monascus Pigments and Monacolin K in Monascus purpureus.: The paper examines how gamma-heptalactone influences the production of bioactive compounds in Monascus purpureus, with implications for food and pharmaceutical industries (Shi et al., 2022).
- RIFM fragrance ingredient safety assessment, gamma-heptalactone, CAS Registry Number 105-21-5.: This safety assessment evaluates gamma-heptalactone as a fragrance ingredient, ensuring its safe use in various consumer products (Api et al., 2019).
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Skin Irrit. 2
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.