P39605
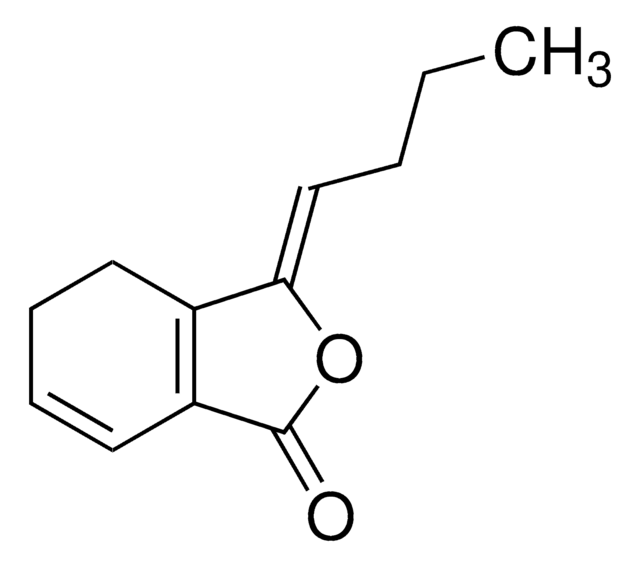
Phthalide
98%
Sinonimo/i:
1-Isobenzofuranone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H6O2
Numero CAS:
Peso molecolare:
134.13
Beilstein:
114632
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
powder
P. ebollizione
290 °C (lit.)
Punto di fusione
71-74 °C (lit.)
Stringa SMILE
O=C1OCc2ccccc12
InChI
1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
WNZQDUSMALZDQF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
305.6 °F - closed cup
Punto d’infiammabilità (°C)
152 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Fangrui Zhong et al.
Journal of the American Chemical Society, 134(24), 10222-10227 (2012-05-25)
Phthalides were used for the first time in the allylic alkylation reactions with MBH carbonates for the creation of chiral 3,3-disubstituted phthalides. Highly enantioselective regiodivergent synthesis of γ-selective or β-selective allylic alkylation products was achieved by employing bifunctional chiral phosphines
Yun Wei et al.
Journal of chromatography. A, 1284, 53-58 (2013-03-15)
The phthalide compounds of Chuanxiong rhizoma including senkyunolide A, levistolide A, Z-ligustilide and 3-butylidenephthalide, have been reported as the biologically active compounds because of their therapeutic effects. In this work, online high-speed counter-current chromatography coupled with semi-preparative liquid chromatography instrument
Bin Xiao et al.
Bioorganic & medicinal chemistry, 20(16), 4954-4961 (2012-07-24)
On the basis of a marine fungal phthalide (paecilocin A) skeleton, we synthesized 20 analogs and evaluated them for peroxisome proliferator-activated receptor gamma (PPAR-γ) binding and activation. Among these analogs, 6 and 7 had significant PPAR-γ binding activity, and 7
Chang-Yin Li et al.
Journal of pharmaceutical and biomedical analysis, 55(1), 146-160 (2011-02-01)
In this work, the metabolite profiles of Danggui Buxue Tang (DBT) in rat bile and plasma were qualitatively described, and the possible metabolic pathways of DBT were subsequently proposed. Emphasis was put on correlative analysis of metabolite profiling in different
Song-Hwa Chae et al.
Journal of agricultural and food chemistry, 59(15), 8193-8198 (2011-07-07)
The residual contact toxicity of three benzofuranoids (Z)-butylidenephthalide (1), (3S)-butylphthalide (2), and (Z)-ligustilide (3) identified in the rhizome of Cnidium officinale (Apiaceae) to B- and Q-biotype females of Bemisia tabaci was evaluated using a leaf-dip bioassay. Results were compared with
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.