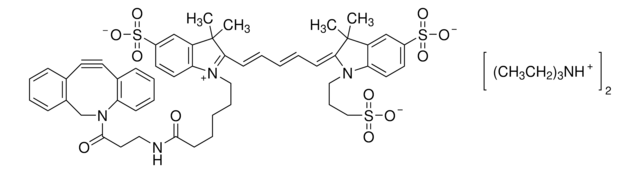
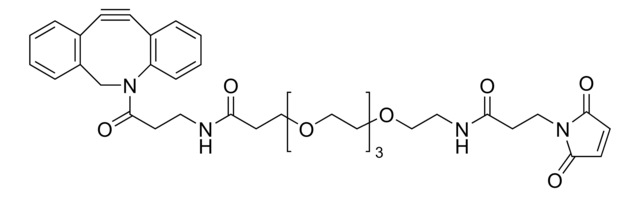
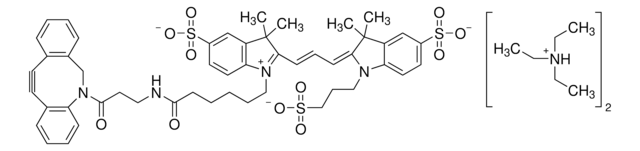
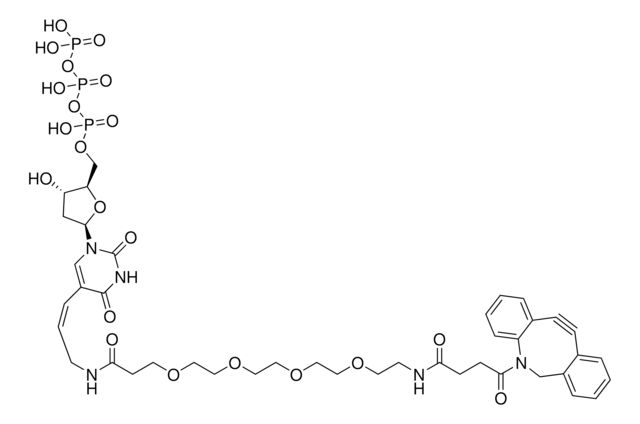
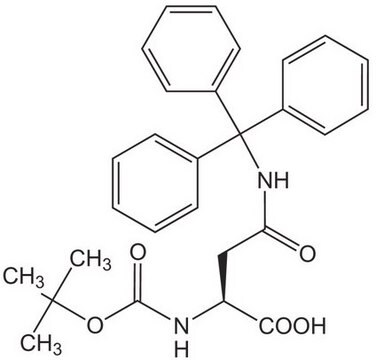
761540
Dibenzocyclooctyne-amine
for Copper-free Click Chemistry
Sinonimo/i:
DBCO-NH2, DBCO-amine
About This Item
Prodotti consigliati
Saggio
≥94.5 %
Stato
solid
Impiego in reazioni chimiche
reaction type: click chemistry
reagent type: linker
Punto di fusione
86-96 °C
Gruppo funzionale
amine
Temperatura di conservazione
−20°C
Stringa SMILE
NCCC(N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3)=O
InChI
1S/C18H16N2O/c19-12-11-18(21)20-13-16-7-2-1-5-14(16)9-10-15-6-3-4-8-17(15)20/h1-8H,11-13,19H2
OCCYFTDHSHTFER-UHFFFAOYSA-N
Descrizione generale
Applicazioni
- Linker to link azide-containing functional groups. It facilitates the formation of linkages through Strain-promoted azide-alkyne cycloaddition reactions (SPAAC)
- Reagent in the synthesis of N-heterocyclic carbene metal thiolates. It functionalizes the metal complexes and increases their reactivity in the strain-promoted alkyne–azide cycloaddition (SPAAC) reactions
- Amine functionalized cyclooctyne derivative. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne cycloaddition reactions. This dibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
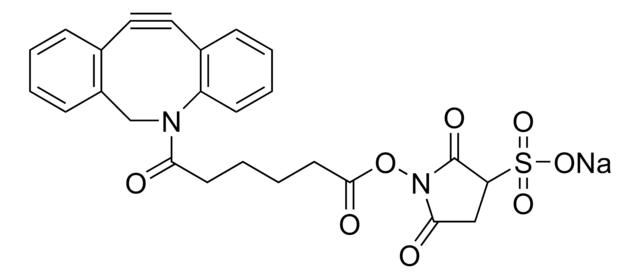
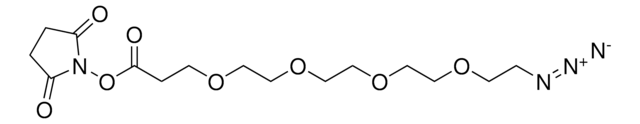
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)
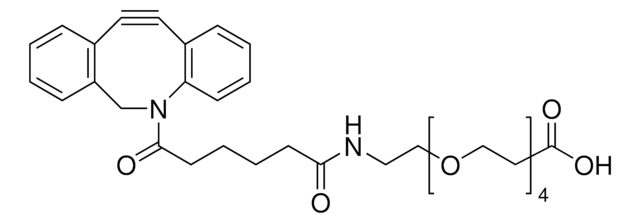
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)
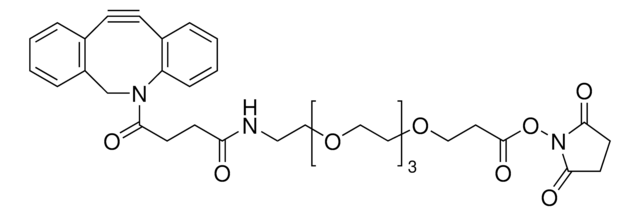
![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)