761516
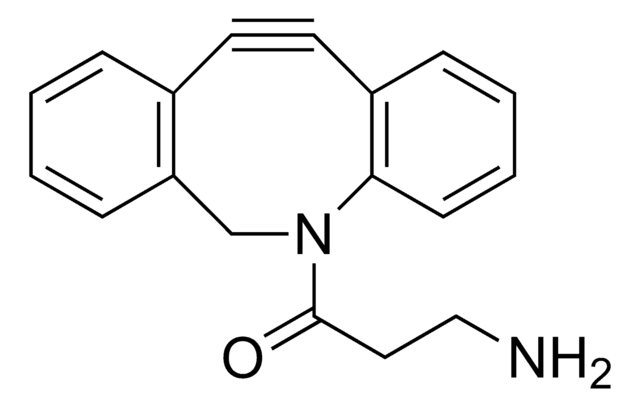
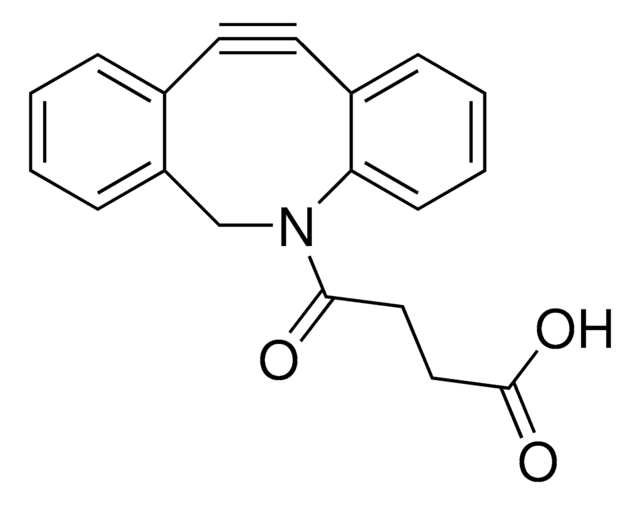
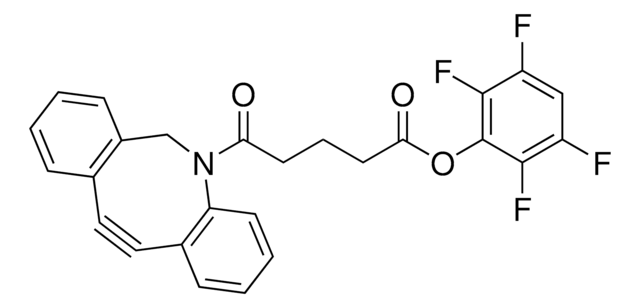
Dibenzocyclooctyne-acid
95%, storage temp.:-20°C
Sinonimo/i:
DBCO-Acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C21H19NO3
Peso molecolare:
333.38
Numero MDL:
Codice UNSPSC:
12352106
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
95%
Stato
solid
Impiego in reazioni chimiche
reaction type: click chemistry
reagent type: linker
Punto di fusione
118-125 °C
Gruppo funzionale
carboxylic acid
Temperatura di conservazione
−20°C
Stringa SMILE
O=C(CCCCC(O)=O)N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3
InChI
1S/C21H19NO3/c23-20(11-5-6-12-21(24)25)22-15-18-9-2-1-7-16(18)13-14-17-8-3-4-10-19(17)22/h1-4,7-10H,5-6,11-12,15H2,(H,24,25)
NIRLBCOFKPVQLM-UHFFFAOYSA-N
Descrizione generale
Acid functionalized cyclooctyne derivative. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne click chemistry reactions. This azadibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Applicazioni
Dibenzocyclooctyne-acid may be used for the surface modification of eight-arm poly(ethylene glycol), to make it susceptible to strain promoted alkyne-azide cycloaddition (SPAAC) with PEG-bis-azide leading to the formation of hydrogels. These hydrogels are useful for protein immobilization.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Bio-orthogonal conjugation and enzymatically triggered release of proteins within multi-layered hydrogels.
Guo C, et al.
Acta Biomaterialia (2017)
Neuroendocrine Tumor?Targeted Upconversion Nanoparticle?Based Micelles for Simultaneous NIR?Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging.
Chen G, et al.
Advances in Functional Materials, 27(8) (2017)
A `catch and release?strategy towards HPLC-free purification of synthetic oligonucleotides by a combination of the strain-promoted alkyne-azide cycloaddition and the photocleavage.
Igata Y, et al.
Bioorganic & Medicinal Chemistry (2017)
Near-infrared light-triggered thermochemotherapy of cancer using a polymer?gold nanorod conjugate.
Ko H, et al.
Nanotechnology, 27(17), 175102-175102 (2016)
Flexible synthesis of cationic peptide?porphyrin derivatives for light-triggered drug delivery and photodynamic therapy.
Dondi R, et al.
Organic & Biomolecular Chemistry, 14(48), 11488-11501 (2016)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.