390704
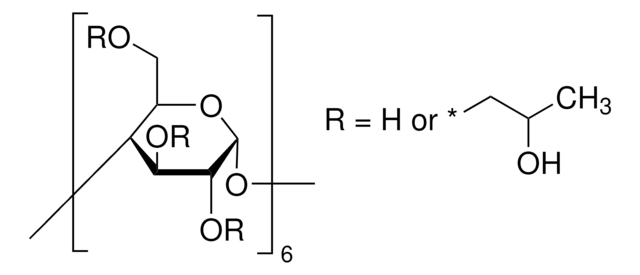
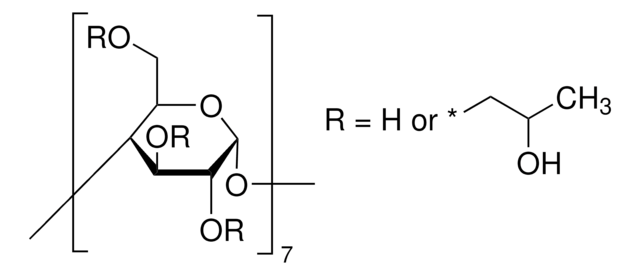
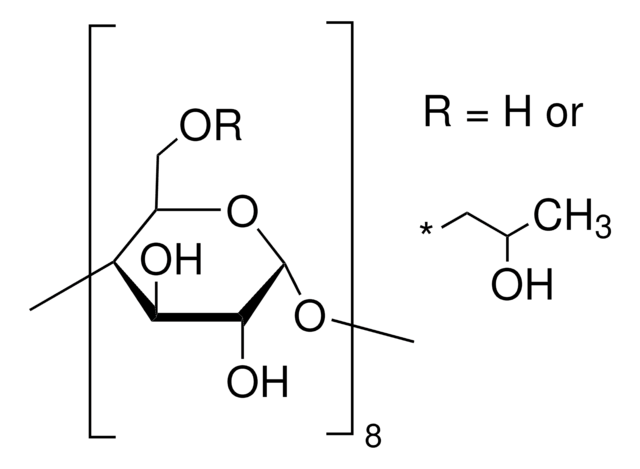
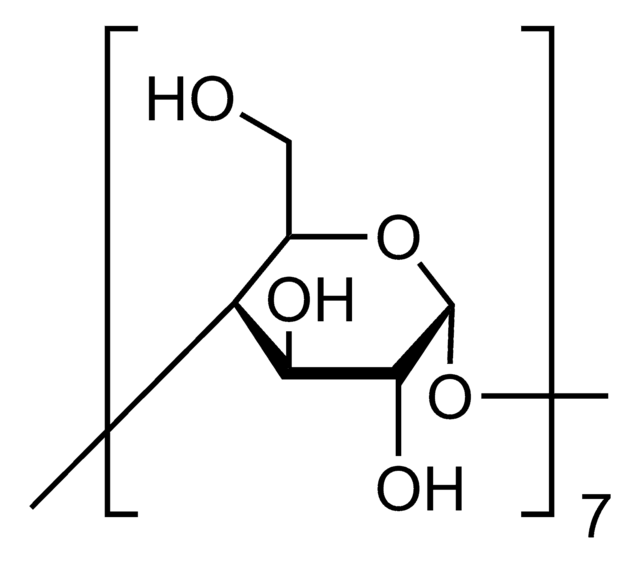
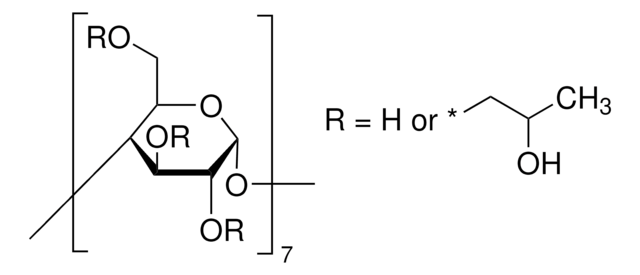
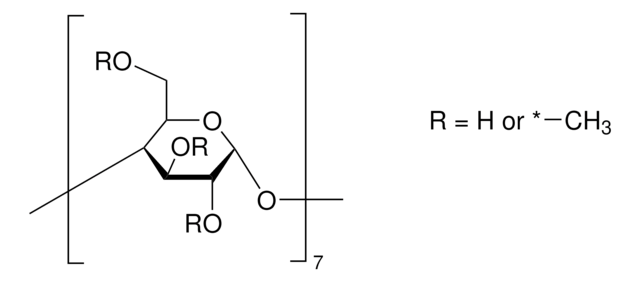
(2-Hydroxypropyl)-γ-cyclodextrin
extent of labeling: 0.6 molar substitution
Sinonimo/i:
HP-γ-CD, HPGCD, HGC
About This Item
Prodotti consigliati
Stato
powder
Livello qualitativo
Attività ottica
[α]20/D +145°, c = 1 in H2O
PM
average Mw ~1,580
Grado di funzionalizzazione
0.6 molar substitution
Stringa SMILE
O1[C@@H]2O[C@H]([C@H](O[C@@H]3O[C@H]([C@H](O[C@@H]4O[C@H]([C@H](O[C@@H]5O[C@H]([C@H](O[C@@H]6O[C@H]([C@H](O[C@@H]7O[C@H]([C@H](O[C@@H]8O[C@H]([C@H]1[C@H]([C@@H]8O)O)COCC(O)C)[C@H]([C@@H]7O)O)CO)[C@H]([C@@H]6O)O)COCC(O)C)[C@H]([C@@H]5O)O)CO)[C@H]([C@@H]4O)
InChI
1S/C51H88O38/c1-14(56)8-73-11-21-42-29(64)36(71)50(81-21)85-40-19(6-54)79-48(34(69)27(40)62)89-44-23(13-75-10-16(3)58)82-51(37(72)30(44)65)86-41-20(7-55)78-47(33(68)26(41)61)88-43-22(12-74-9-15(2)57)80-49(35(70)28(43)63)84-39-18(5-53)76-45(31(66)24(39)59)83-38-17(4-52)77-46(87-42)32(67)25(38)60/h14-72H,4-13H2,1-3H3/t14?,15?,16?,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1
ODLHGICHYURWBS-RYJYQAAZSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- As a mobile phase additive in the study of the host-guest interaction with organic low molecular mass compounds prior to their quantification using reversed phase-high performance liquid chromatography (RP-HPLC) technique.
- As a chiral surfactant for the analysis of econazole by micellar electrokinetic chromatography (MEKC).
- As an analytical standard for the determination of the analyte in biological samples by HPLC.
- As a chiral selector for the identification of propiconazole by MEKC.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.