S2500
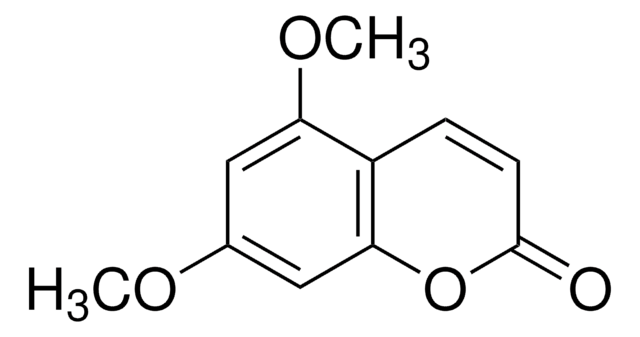
Scopoletin
≥99%
Sinônimo(s):
6-Methoxyumbelliferone, 6-Methylesculetin, 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one, 7-Hydroxy-6-methoxycoumarin, Buxuletin, Chrysatropic acid, Escopoletin, Esculetin-6-methyl ether, Gelseminic acid, Murrayetin
About This Item
Produtos recomendados
Ensaio
≥99%
pf
203-205 °C (lit.)
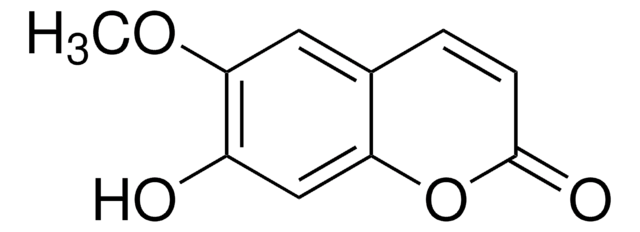
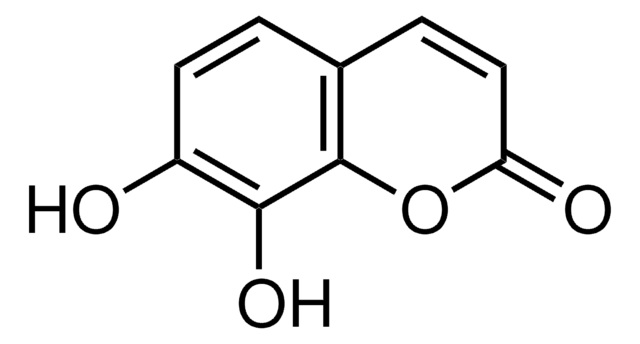
cadeia de caracteres SMILES
COc1cc2C=CC(=O)Oc2cc1O
InChI
1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3
chave InChI
RODXRVNMMDRFIK-UHFFFAOYSA-N
Informações sobre genes
human ... ACHE(43)
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Production of Polyphenolic Natural Products by Bract-Derived Tissue Cultures of Three Medicinal Tilia spp.: A Comparative Untargeted Metabolomics Study.: This study investigates the production of polyphenolic compounds, including scopoletin, in tissue cultures derived from the bracts of three medicinal Tilia species. The research highlights the potential of these cultures in producing valuable natural products (Szűcs et al., 2024).
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica