116238
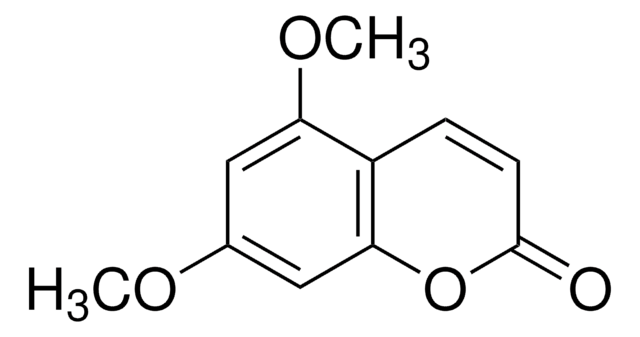
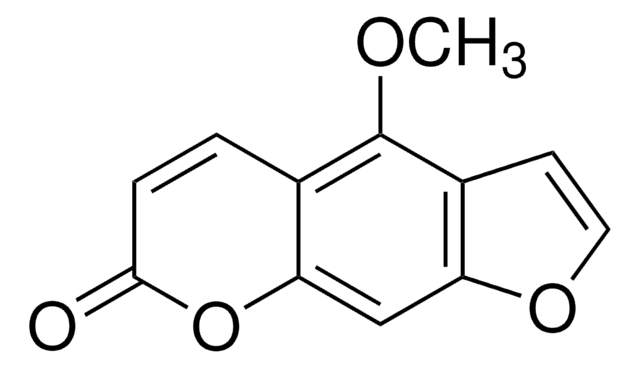
5,7-Dimethoxycoumarin
98%
Sinônimo(s):
Citropten, Limettin
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C11H10O4
Número CAS:
Peso molecular:
206.19
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
Formulário
solid
pf
146-149 °C (lit.)
grupo funcional
ester
cadeia de caracteres SMILES
COc1cc(OC)c2C=CC(=O)Oc2c1
InChI
1S/C11H10O4/c1-13-7-5-9(14-2)8-3-4-11(12)15-10(8)6-7/h3-6H,1-2H3
chave InChI
NXJCRELRQHZBQA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
5,7-dimethoxycoumarin is isolated and identified from leaves and fruits of Pelea anisata H. Mann, a plant whose fruit are used in the construction of mohikana leis. It induces frameshift mutagenesis in bacteria. It also causes lethal photosensitization and the formation of sister chromatid exchanges in Chinese hamster cells.
Ações bioquímicas/fisiológicas
5,7-Dimethoxycoumarin induces the processes of differentiation and melanogenesis in murine (B16) and human (A375).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Daniela Alesiani et al.
International journal of oncology, 32(2), 425-434 (2008-01-19)
In the present study we investigated the antiproliferative activity of 5,7-dimethoxycoumarin on the murine B16 and human A375 melanoma cell lines. The inhibitory concentration 50 (IC50) was estimated for each cell line by preliminary assay of tetrazolium salt reduction (MTT).
Earl Grey tea intoxication.
Josef Finsterer
Lancet (London, England), 359(9316), 1484-1484 (2002-05-04)
Ewa Chodurek et al.
Cellular & molecular biology letters, 17(4), 616-632 (2012-09-25)
Malignant melanoma (melanoma malignum) is one of the most dangerous types of tumor. It is very difficult to cure. In recent years, a lot of attention has been given to chemoprevention. This method uses natural and synthetic compounds to interfere
D Chouchi et al.
Journal of chromatography. A, 672(1-2), 177-183 (1994-06-24)
Generally on the gas chromatogram of a volatile essential oil, terpenes, oxygenated compounds and sesquiterpenes appear. With temperature programming, it was shown that some non-volatiles are present with the volatiles. They are simple coumarin (2H-1-benzopyran-2-one) derivatives such as citropten (5,7-dimethoxycoumarin)
Tomonori Nakamura et al.
Journal of natural medicines, 63(1), 15-20 (2008-07-09)
We have investigated the structure-activity relationship between 63 natural oxycoumarin derivatives and their effects on the expression of inducible-nitric oxide synthase (iNOS) induced by lipopolysaccharide. The protein expression of iNOS was screened by Western blot analysis, and four 5,7-dimethoxycoumarins were
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica