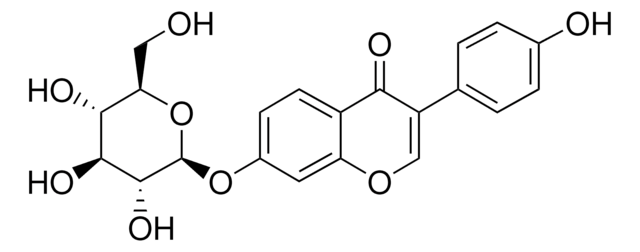
G1296
Glycitin
≥98% (HPLC)
Sinônimo(s):
4′,7-Dihydroxy 6-methoxyisoflavone 7-O-glucoside, 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-3-(4-hydroxyphenyl)-6-methoxy-, Glycitein 7-O-β-glucoside
About This Item
Produtos recomendados
fonte biológica
plant (Pueraria thunbergianaI)
Nível de qualidade
Ensaio
≥98% (HPLC)
forma
powder
técnica(s)
HPLC: suitable
cor
white to faint beige
pf
203 - 204 °C ((397 - 399 °F ))
solubilidade
10 mg, clear, colorless to faintly yellow
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
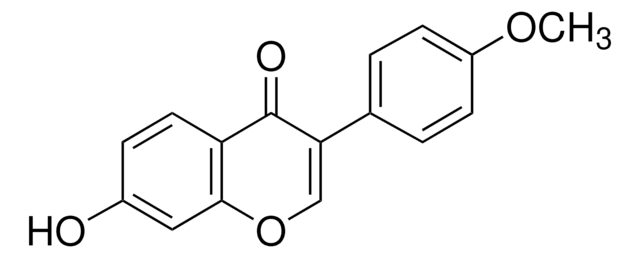
O=C1C2=CC(OC)=C(O[C@H]3[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O3)C=C2OC=C1C4=CC=C(O)C=C4
InChI
1S/C22H22O10/c1-29-15-6-12-14(30-9-13(18(12)25)10-2-4-11(24)5-3-10)7-16(15)31-22-21(28)20(27)19(26)17(8-23)32-22/h2-7,9,17,19-24,26-28H,8H2,1H3/t17-,19-,20+,21-,22-/m1/s1
chave InChI
OZBAVEKZGSOMOJ-MIUGBVLSSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells.: This study highlights Glycitin′s therapeutic potential in mitigating intervertebral disc degeneration by counteracting inflammatory and oxidative processes, demonstrating its viability as a bioactive compound for degenerative diseases (Zhao W et al., 2023).
- Spectrum-effect relationship study to reveal the pharmacodynamic substances in Flos Puerariae-Semen Hoveniae medicine pair for the treatment of alcohol-induced liver damage.: Analyzes the pharmacological effects of herbal components, including Glycitin, targeting liver damage recovery, illustrating the compound′s therapeutic relevance in traditional and modern medical applications (Zhang H et al., 2023).
Ações bioquímicas/fisiológicas
Embalagem
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica