A8054
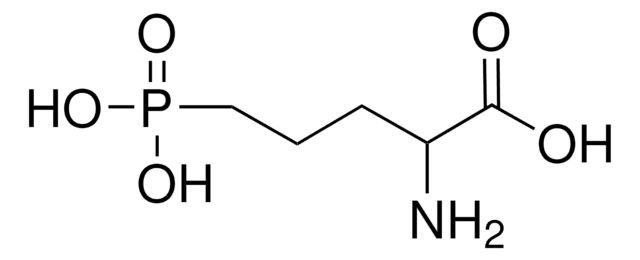
D(−)-2-Amino-5-phosphonopentanoic acid
NMDA receptor antagonist
Sinônimo(s):
D(−)-AP-5, D(−)-APV, D-2-Amino-5-phosphonovaleric acid
About This Item
Produtos recomendados
Ensaio
≥98% (TLC)
forma
powder
pureza óptica
optical purity: ≥90% (HPLC, Marfey′s reagent)
técnica(s)
ligand binding assay: suitable
cor
white
pf
245-246 °C
cadeia de caracteres SMILES
N[C@H](CCCP(O)(O)=O)C(O)=O
InChI
1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11)/t4-/m1/s1
chave InChI
VOROEQBFPPIACJ-SCSAIBSYSA-N
Informações sobre genes
mouse ... Grin2a(14811)
rat ... Grik1(29559) , Grin2a(24409) , Grin2b(24410) , Grin2c(24411) , Grin2d(24412)
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Rats and Blockade of Long-Term Potentiation in viva by the
IV-Methyl-D-Aspartate Receptor Antagonist AP5
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica