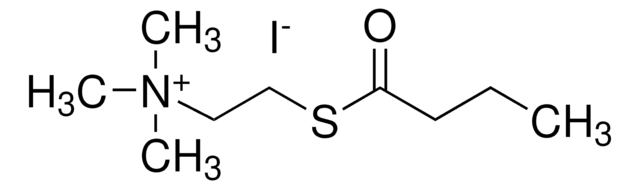
A7000
Acetylcholine iodide
≥97%
Sinônimo(s):
ACh
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(CH3)3N(I)CH2CH2OCOCH3
Número CAS:
Peso molecular:
273.11
Beilstein:
3571339
Número CE:
Número MDL:
Código UNSPSC:
12352107
ID de substância PubChem:
NACRES:
NA.25
Produtos recomendados
Nível de qualidade
Ensaio
≥97%
Formulário
powder
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
[I-].CC(=O)OCC[N+](C)(C)C
InChI
1S/C7H16NO2.HI/c1-7(9)10-6-5-8(2,3)4;/h5-6H2,1-4H3;1H/q+1;/p-1
chave InChI
SMBBQHHYSLHDHF-UHFFFAOYSA-M
Informações sobre genes
human ... CHRM3(1131)
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Acetylcholine is an endogenous neurotransmitter at cholinergic synapses that amplifies action potential of the sarcolemma thereby inducing muscle contractions. Acetylcholine iodide is used as an acetylcholine receptor agonist to identify, characterize and differentiate among types of cholinergic receptors. Acetylcholine iodide is used as a substrate to identify and characterize natural and mutated acetylcholinesterase(s).
Ações bioquímicas/fisiológicas
Endogenous neurotransmitter at cholinergic synapses; amplifies action potential of the sarcolemma thereby inducing muscle contractions.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Iryna Andrusenko et al.
Angewandte Chemie (International ed. in English), 58(32), 10919-10922 (2019-06-19)
Orthocetamol is a regioisomer of the well-known pain medication paracetamol and a promising analgesic and an anti-arthritic medicament itself. However, orthocetamol cannot be grown as single crystals suitable for X-ray diffraction, so its crystal structure has remained a mystery for
Marie Briet et al.
Journal of the American Heart Association, 2(2), e000128-e000128 (2013-04-16)
Recent studies have raised concern about the safety of erythropoiesis-stimulating agents because of evidence of increased risk of hypertension and cardiovascular morbidity and mortality in chronic kidney disease (CKD) patients. In the present study, we investigated the effects of recombinant
Tracey Huynh et al.
Journal of medicinal chemistry, 56(20), 8196-8200 (2013-10-01)
The M4 mAChR is implicated in several CNS disorders and possesses an allosteric binding site for which ligands modulating the affinity and/or efficacy of ACh may be exploited for selective receptor targeting. We report the synthesis of a focused library
Wei Wang et al.
Journal of biomedical nanotechnology, 9(4), 736-740 (2013-04-30)
A novel type of acetylcholine electrochemical biosensor was successfully fabricated by dropping beta-cyclodextrin solution, acetylcholinesterase solution and Nafion-methanol solution to the homemade nano-porous pseudo carbon paste electrode (nano-PPCPE) as working electrode, and then the electrochemical behavior of the as-prepared biosensor
Carolina Wedemeyer et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(39), 15477-15487 (2013-09-27)
The synapse between olivocochlear (OC) neurons and cochlear mechanosensory hair cells is cholinergic, fast, and inhibitory. The inhibitory sign of this cholinergic synapse is accounted for by the activation of Ca(2+)-permeable postsynaptic α9α10 nicotinic receptors coupled to the opening of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica