22620
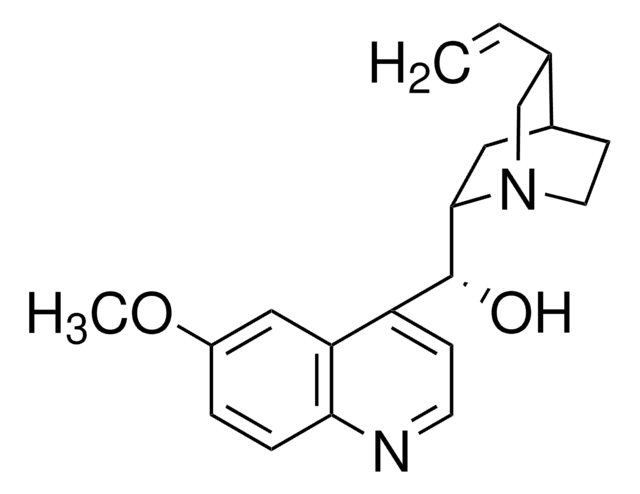
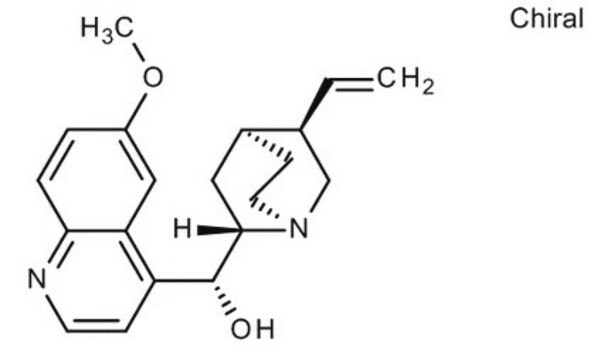
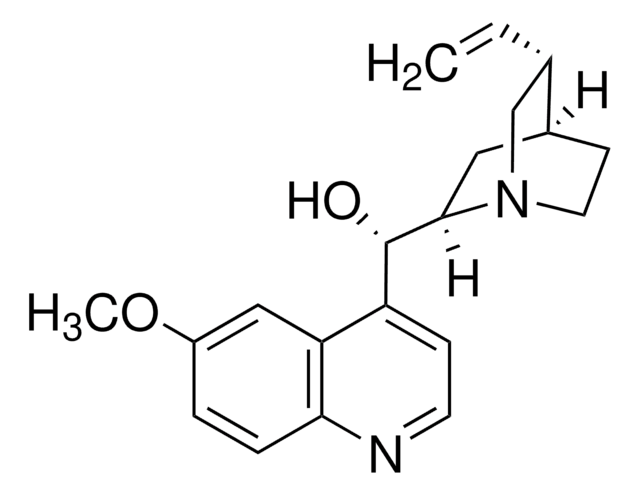
Quinine
suitable for fluorescence, anhydrous, ≥98.0% (dried material, NT)
Sinônimo(s):
6′-Methoxycinchonidine
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98.0% (dried material, NT)
Formulário
powder
atividade óptica
[α]20/D −126±5°, c = 1% in chloroform
Impurezas
≤5% dihydroquinine (HPLC)
perda
≤1% loss on drying, 110 °C
pf
173-175 °C (lit.)
solubilidade
H2O: soluble
fluorescência
λex 347 nm; λem 448 nm in 0.5 M sulfuric acid
adequação
suitable for fluorescence
cadeia de caracteres SMILES
COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1
InChI
1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1
chave InChI
LOUPRKONTZGTKE-WZBLMQSHSA-N
Informações sobre genes
human ... ABCB1(5243) , CYP2C9(1559) , CYP2D6(1565)
rat ... Cyp2d1(266684) , Cyp2d2(25053) , Cyp2d3(24303) , Cyp2d4v1(171522)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- To study its in vitro antimalarial activity in combination with omeprazole.
- To analyze its effect on viscosity and friction of saliva.
- As a test agent to study its impact on the accumulation of the fluorescent P-glycoprotein (Pgp) substrates in P-glycoprotein overexpressing breast cancer cells.
- To study its influence on the pyramidal cell intrinsic properties, extracellular potassium transients, and epileptiform activity in vitro.
- As a reference compound to identify alkaloids by phytochemical screening of Deianira erubescens, Strychnos pseudoquina and Remijia ferruginea plants.
Ações bioquímicas/fisiológicas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica