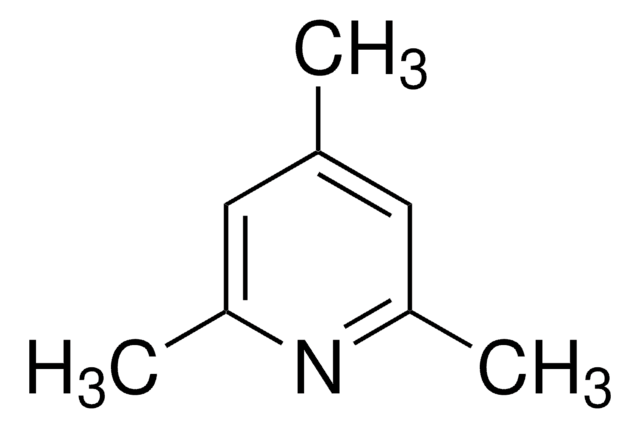
L3900
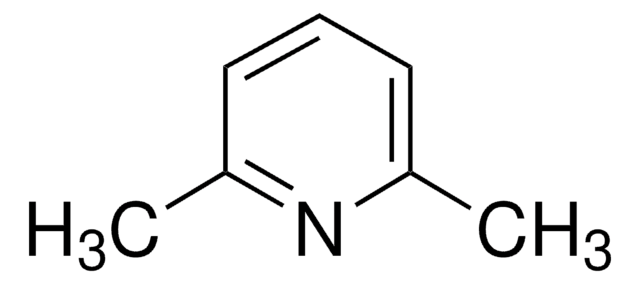
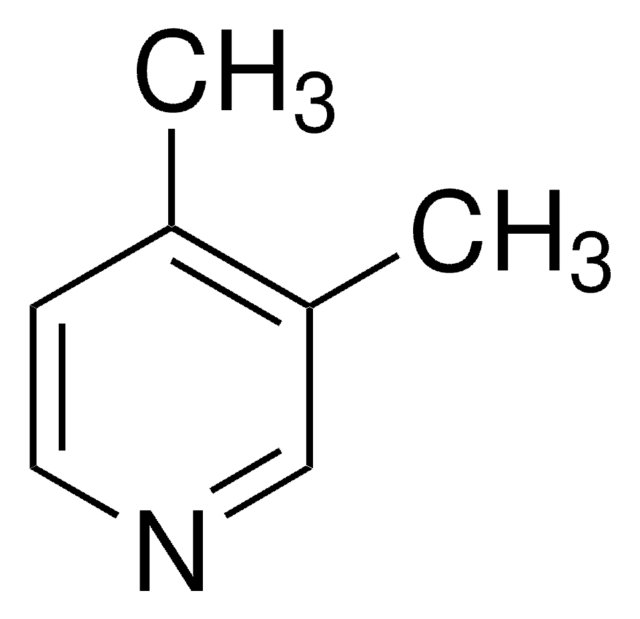
2,6-Lutidina
ReagentPlus®, 98%
Sinônimo(s):
2,6-Dimetilpiridina
About This Item
Produtos recomendados
Nível de qualidade
linha de produto
ReagentPlus®
Ensaio
98%
índice de refração
n20/D 1.497 (lit.)
p.e.
143-145 °C (lit.)
pf
−6 °C (lit.)
densidade
0.92 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Cc1cccc(C)n1
InChI
1S/C7H9N/c1-6-4-3-5-7(2)8-6/h3-5H,1-2H3
chave InChI
OISVCGZHLKNMSJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
It can be used:
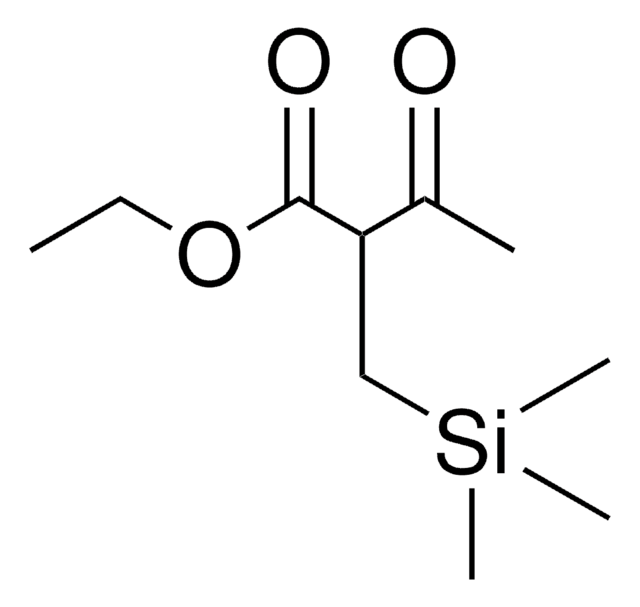
- As a promoter for catalytic asymmetric fluorination of α-cyanophosphonates in the presence of chiral Pd(II)-bisphosphine complexes.
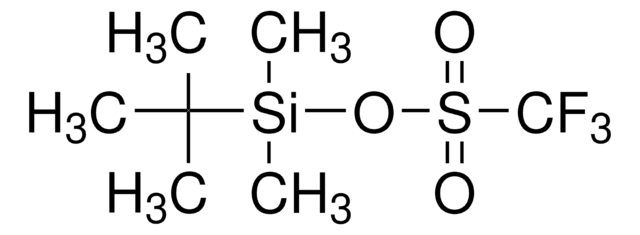
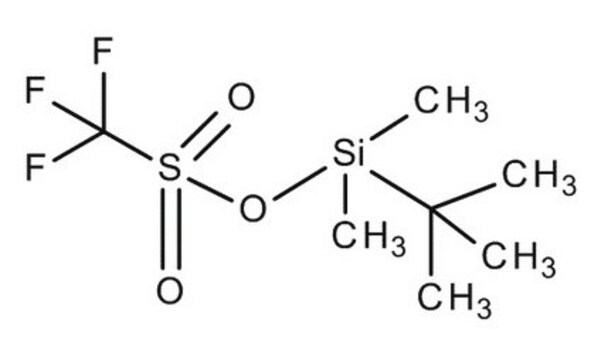
- In combination with tert-butyldimethylsilyl triflate for the protection of tertiary alcohols and unreactive secondary alcohols.
- In combination with triethylsilyl trifluoromethanesulfonate for the conversion of acetals to the corresponding aldehydes in dichloromethane followed by workup in water.
Informações legais
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
91.4 °F
Ponto de fulgor (°C)
33 °C
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica