D152927
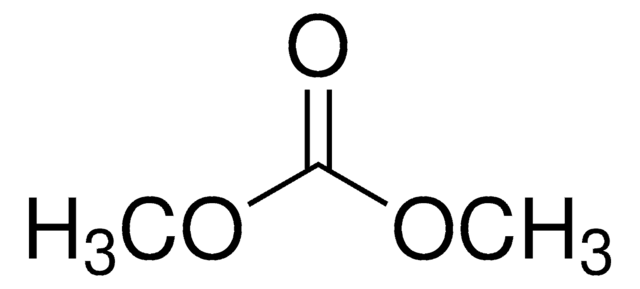
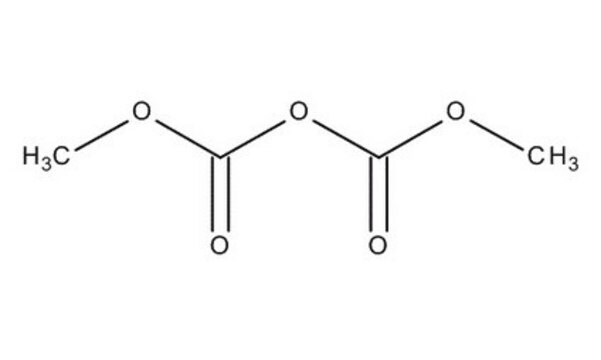
Dimethyl carbonate
ReagentPlus®, 99%
Sinônimo(s):
Carbonic acid dimethyl ester
About This Item
Produtos recomendados
grau
reagent
Nível de qualidade
densidade de vapor
3.1 (vs air)
pressão de vapor
18 mmHg ( 21.1 °C)
linha de produto
ReagentPlus®
Ensaio
99%
Formulário
liquid
características do produto alternativo mais ecológico
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
dilution
(for general lab use)
índice de refração
n20/D 1.368 (lit.)
p.e.
90 °C (lit.)
pf
2-4 °C (lit.)
densidade
1.069 g/mL at 25 °C (lit.)
categoria alternativa mais ecológica
, Aligned
cadeia de caracteres SMILES
O=C(OC)OC
InChI
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3
chave InChI
IEJIGPNLZYLLBP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a Greener alternative to conventional solvents and chemicals. Click here for more information.
Aplicação
- Methyl phenyl carbonate by transesterification with phenol.
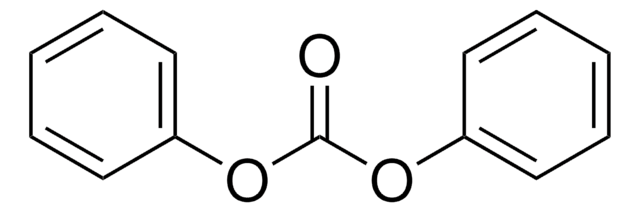
- Diphenyl carbonate by transesterification with methyl phenyl carbonate.
- Methyl carbamates, a raw material for isocyanate synthesis.
- Tetramethoxysilane by reacting with silica at 550-600K.
Características e benefícios
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
60.8 °F - closed cup
Ponto de fulgor (°C)
16 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica