86040
Sulfamic acid
analytical standard (for acidimetry), ACS reagent
Sinônimo(s):
Aminosulfonic acid, Sulphamic acid, Amidosulfonic acid
About This Item
Produtos recomendados
grau
ACS reagent
analytical standard (for acidimetry)
Nível de qualidade
Agency
complies with DIN 19266
Ensaio
99.3-100.3% (dried material)
pf
215-225 °C (dec.) (lit.)
traços de ânion
chloride (Cl-): ≤10 mg/kg
sulfate (SO42-): ≤500 mg/kg
traços de cátion
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
K: ≤50 mg/kg
Mg: ≤5 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤10 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
aplicação(ões)
environmental
food and beverages
general analytical
industrial qc
pharmaceutical
Formato
mixture
cadeia de caracteres SMILES
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
chave InChI
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Sulfamic acid also finds its use as a primary standard in non-aqueous visual, conductometric, and potentiometric titrations.
Características e benefícios
- Available in a secure glass bottle to ensure its stability for the entire shelf life until opened.
- High-quality offering accurate titer determinations
- Accompanied by a detailed certificate of analysis (CoA)
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica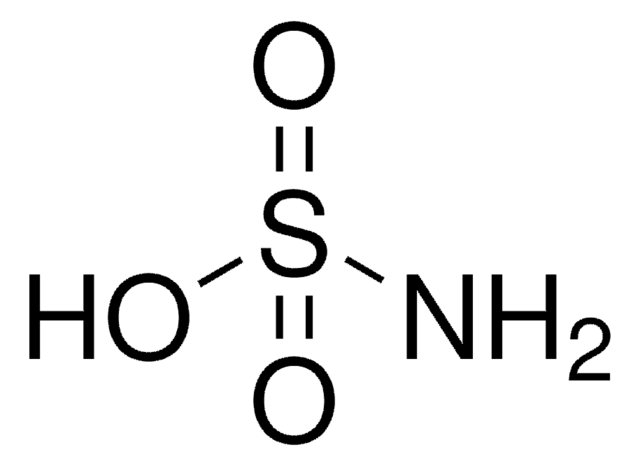


![[(3R)-3-Hydroxytetradecanoyl]-L-carnitine-(N-methyl-d3) analytical standard](/deepweb/assets/sigmaaldrich/product/structures/359/244/1ce23698-2525-447b-8fab-501b73a8c5da/640/1ce23698-2525-447b-8fab-501b73a8c5da.png)