1.00489
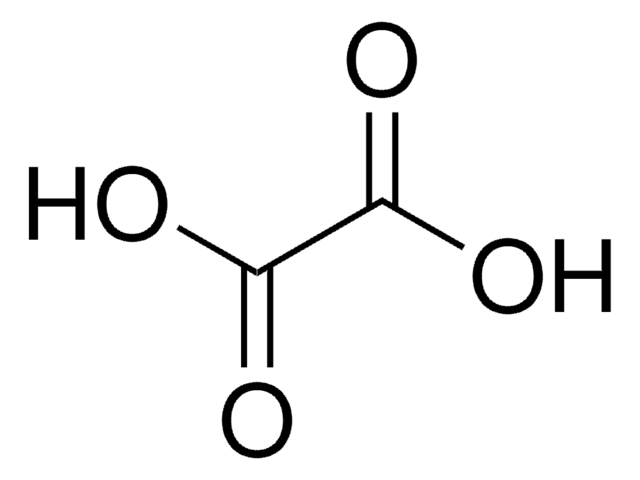
Oxalic acid dihydrate
Suprapur®, for inorganic trace analysis
Sinônimo(s):
Oxalic acid dihydrate, Ethanedioic acid
About This Item
Produtos recomendados
grau
for inorganic trace analysis
Nível de qualidade
pressão de vapor
0.000312 hPa ( 25 °C)
Ensaio
≥99.5% (calculated as dihydrate, alkalimetric)
Formulário
solid
potência
375 mg/kg LD50, oral (Rat)
pH
1.5 (10 g/L in H2O)
p.e.
149-160 °C/1013 hPa (decomposition)
pf
98-100 °C
temperatura de transição
flash point 157 °C (decomposition)
solubilidade
water: >100 g/L at 25 °C
densidade
1.65 g/cm3 at 20 °C
densidade volumétrica
813 kg/m3
traços de ânion
chloride (Cl-): ≤5000 ppb
phosphate (PO43-): ≤500 ppb
sulfate (SO42-): ≤2000 ppb
traços de cátion
Ag: ≤10 ppb
Al: ≤20 ppb
As: ≤1.0 ppb
Au: ≤1.0 ppb
Ba: ≤100 ppb
Be: ≤1.0 ppb
Bi: ≤1.0 ppb
Ca: ≤100 ppb
Cd: ≤50 ppb
Co: ≤5 ppb
Cr: ≤10 ppb
Cu: ≤5 ppb
Fe: ≤50 ppb
Ga: ≤1.0 ppb
Ge: ≤1.0 ppb
In: ≤1.0 ppb
K: ≤200 ppb
Li: ≤5 ppb
Mg: ≤20 ppb
Mn: ≤5 ppb
Mo: ≤5 ppb
Na: ≤100 ppb
Ni: ≤10 ppb
Pb: ≤10 ppb
Pt: ≤1.0 ppb
Sb: ≤1.0 ppb
Sn: ≤5 ppb
Sr: ≤100 ppb
Ti: ≤5 ppb
Tl: ≤1.0 ppb
U: ≤1.0 ppb
V: ≤10 ppb
Zn: ≤20 ppb
cadeia de caracteres SMILES
[O+H3].[O+H3].[O-]C(=O)C(=O)[O-]
temperatura de armazenamento
2-30°C
InChI
1S/C2H2O4.2H2O/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);2*1H2
chave InChI
GEVPUGOOGXGPIO-UHFFFAOYSA-N
Aplicação
- Reaction Atmosphere-Controlled Thermal Conversion of Ferrocene to Hematite and Cementite Nanomaterials-Structural and Spectroscopic Investigations.: This study investigates the conversion of ferrocene to hematite and cementite nanomaterials under controlled thermal conditions. The structural and spectroscopic properties of the resulting materials were analyzed, providing insights into their potential applications in various fields of chemistry and materials science (Kundu et al., 2024).
- Insights into a Co-precursor Driven Solid-State Thermal Reaction of Ferrocene Carboxaldehyde Leading to Hematite Nanomaterial: A Reaction Kinetic Study.: This research focuses on the solid-state thermal reaction of ferrocene carboxaldehyde with oxalic acid dihydrate, leading to hematite nanomaterial. The study provides detailed reaction kinetics and potential applications in catalysis and materials science (Chakraborty et al., 2023).
- Supramolecular Structure of Microwave Treated Bamboo for Production of Lignin-Containing Nanocellulose by Oxalic Acid Dihydrate.: The paper explores the use of oxalic acid dihydrate in the microwave treatment of bamboo to produce lignin-containing nanocellulose. This process enhances the material properties of nanocellulose, offering applications in sustainable materials and bioengineering (Wang et al., 2023).
- Thermo-Mechano-Chemical Deconstruction of Cellulose for Cellulose Nanocrystal Production by Reactive Processing.: This study presents a method for producing cellulose nanocrystals using thermo-mechano-chemical deconstruction with oxalic acid dihydrate. The resulting nanocrystals have potential uses in biocomposites and nanomaterials (Guiao et al., 2022).
- Formation and Structure Evolution of Starch Nanoplatelets by Deep Eutectic Solvent of Choline Chloride/Oxalic Acid Dihydrate Treatment.: This research investigates the formation of starch nanoplatelets using a deep eutectic solvent comprising choline chloride and oxalic acid dihydrate. The study highlights the structural evolution and potential applications in food science and materials engineering (Xiao et al., 2022).
Nota de análise
Chloride (Cl): ≤ 5000 ppb
Phosphate (PO₄): ≤ 500 ppb
Sulfate (SO₄): ≤ 2000 ppb
Ag (Silver): ≤ 10 ppb
Al (Aluminium): ≤ 20 ppb
As (Arsenic): ≤ 1.0 ppb
Au (Gold): ≤ 1.0 ppb
Ba (Barium): ≤ 100 ppb
Be (Beryllium): ≤ 1.0 ppb
Bi (Bismuth): ≤ 1.0 ppb
Ca (Calcium): ≤ 100 ppb
Cd (Cadmium): ≤ 50 ppb
Co (Cobalt): ≤ 5 ppb
Cr (Chromium): ≤ 10 ppb
Cu (Copper): ≤ 5 ppb
Fe (Iron): ≤ 50 ppb
Ga (Gallium): ≤ 1.0 ppb
Ge (Germanium): ≤ 1.0 ppb
In (Indium): ≤ 1.0 ppb
K (Potassium): ≤ 200 ppb
Li (Lithium): ≤ 5 ppb
Mg (Magnesium): ≤ 20 ppb
Mn (Manganese): ≤ 5 ppb
Mo (Molybdenum): ≤ 5 ppb
Na (Sodium): ≤ 100 ppb
Ni (Nickel): ≤ 10 ppb
Pb (Lead): ≤ 10 ppb
Pt (Platinum): ≤ 1.0 ppb
Sb (Antimony): ≤ 1.0 ppb
Sn (Tin): ≤ 5 ppb
Sr (Strontium): ≤ 100 ppb
Ti (Titanium): ≤ 5 ppb
Tl (Thallium): ≤ 1.0 ppb
U (Uranium): ≤ 1.0 ppb
V (Vanadium): ≤ 10 ppb
Zn (Zinc): ≤ 20 ppb
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica