W323713
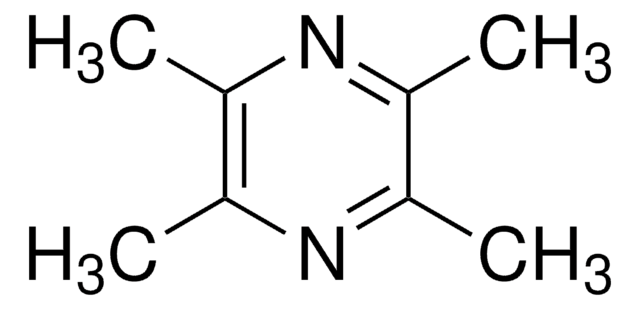
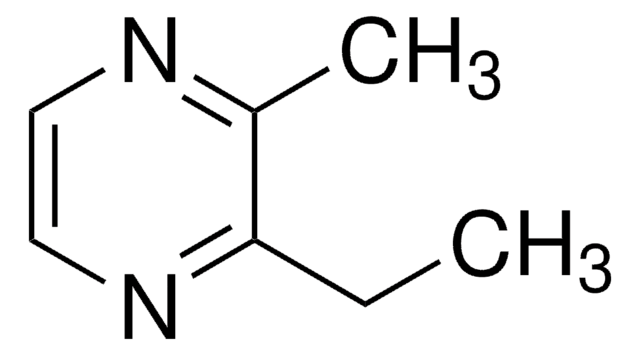
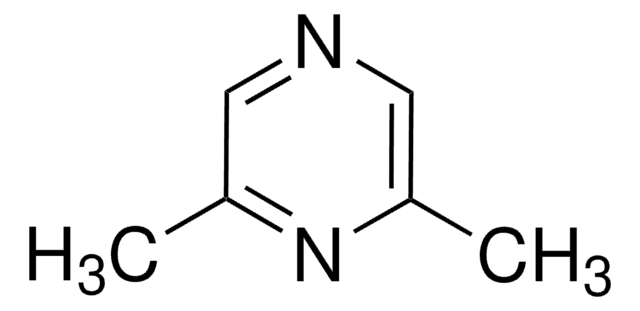
2,3,5,6-Tetramethylpyrazine
natural, ≥98%, FG
Sinônimo(s):
Chuanxingzine, Ligustrazine, Tetrapyrazine
About This Item
Produtos recomendados
grau
FG
Fragrance grade
Halal
Kosher
natural
Agency
follows IFRA guidelines
meets purity specifications of JECFA
conformidade reg.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Ensaio
≥98%
características do produto alternativo mais ecológico
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezas
≤2.0% water (Karl Fischer)
p.e.
190 °C (lit.)
pf
77-80 °C (lit.)
aplicação(ões)
flavors and fragrances
Documentação
see Safety & Documentation for available documents
alérgeno alimentar
no known allergens
alérgeno de fragrância
no known allergens
categoria alternativa mais ecológica
, Aligned
Organoléptico
chocolate; coffee; fatty; musty; nutty
cadeia de caracteres SMILES
Cc1nc(C)c(C)nc1C
InChI
1S/C8H12N2/c1-5-6(2)10-8(4)7(3)9-5/h1-4H3
chave InChI
FINHMKGKINIASC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Booklice Liposcelis bostrychophila are efficiently attracted by the combination of 2,3,5,6-tetramethylpyrazine and ultraviolet light.: This study demonstrates that the combination of 2,3,5,6-tetramethylpyrazine and ultraviolet light effectively attracts booklice, suggesting a potential application for pest management in stored product environments (Tanaka et al., 2024).
- 2,3,5,6-Tetramethylpyrazine protects retinal photoreceptors against endoplasmic reticulum stress by modulating ATF4-mediated inhibition of PRP aggregation.: The research highlights the neuroprotective effects of 2,3,5,6-tetramethylpyrazine, showing its potential in treating retinal diseases by protecting photoreceptors from stress-induced damage (Huang et al., 2021).
- Tetramethylpyrazine-Inducible Promoter Region from Rhodococcus jostii TMP1.: The study identifies a promoter region in Rhodococcus jostii TMP1 that is inducible by tetramethylpyrazine, which could be utilized in genetic engineering and biotechnology applications (Stanislauskienė et al., 2018).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W323713-100G | |
| W323713-SAMPLE-K | 4061834356172 |
| W323713-100G-K | 4061834405672 |
| W323713-1KG | |
| W323713-1KG-K | 4061837528392 |
| W323713-25G | |
| W323713-25G-K | |
| W323713-SAMPLE |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica