P73404
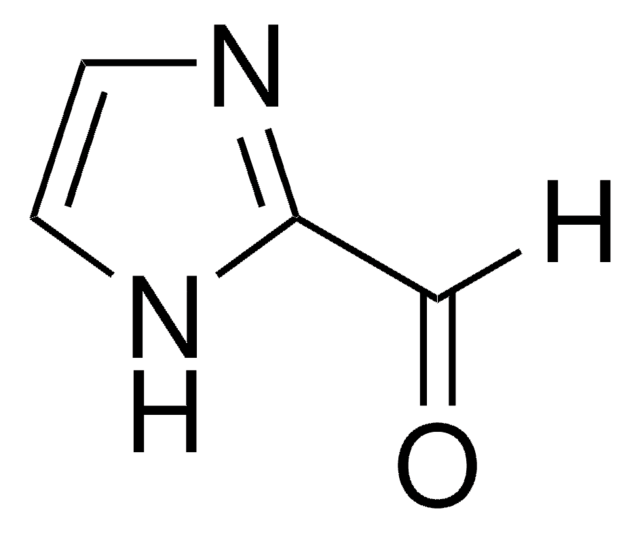
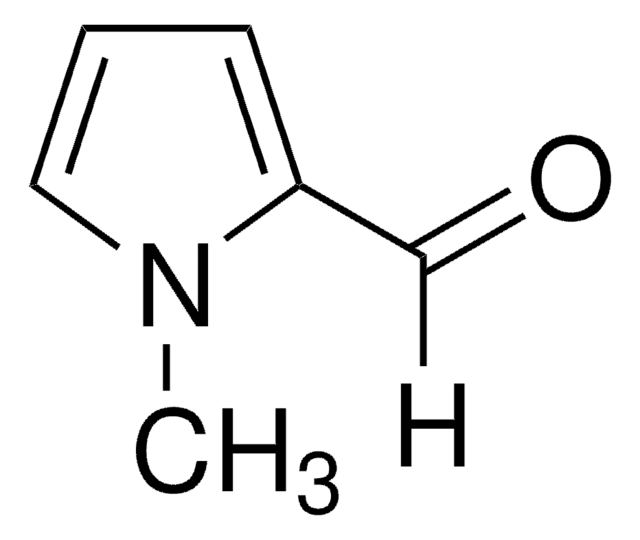
Pyrrole-2-carboxaldehyde
98%
Sinônimo(s):
2-Formylpyrrole
About This Item
Produtos recomendados
Ensaio
98%
Formulário
crystals
p.e.
217-219 °C (lit.)
pf
43-46 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
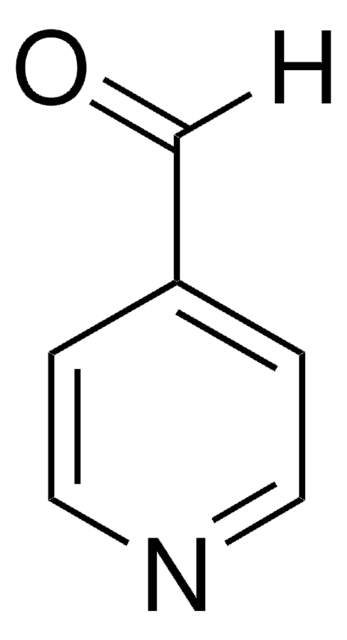
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
chave InChI
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
224.6 °F - closed cup
Ponto de fulgor (°C)
107 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica