M1407
Malononitrile
≥99%
Sinônimo(s):
Dicyanomethane
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥99%
pb
220 °C (lit.)
pf
30-32 °C (lit.)
densidade
1.049 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
chave InChI
CUONGYYJJVDODC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- base-promoted on-water synthesis of [1,6]-naphthyridines.†
- synthesis of γ-ketoamides.
- preparation of heterocyclic privileged medicinal scaffolds involving pyridine, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
Embalagem
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
186.8 °F - closed cup
Ponto de fulgor (°C)
86 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica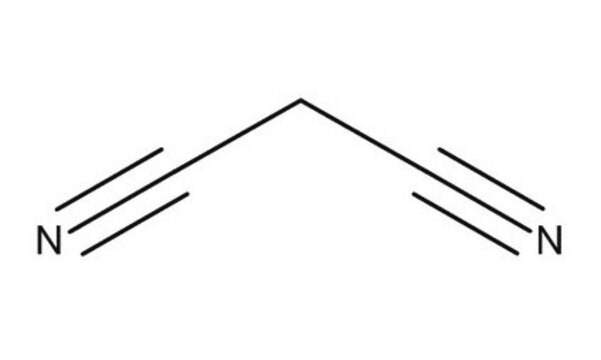







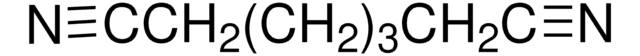
![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)