E18425
Ethyl cyanoacetate
≥98%
Sinônimo(s):
(Ethoxycarbonyl)acetonitrile, 2-Cyanoacetic acid ethyl ester, 3-Ethoxy-3-oxopropanenitrile, Cyanoacetic acid ethyl ester, Cyanoacetic ester, Ethyl α-cyanoacetate, Ethyl 2-cyanoacetate, Ethyl cyanacetate, Malonic acid ethyl ester nitrile
About This Item
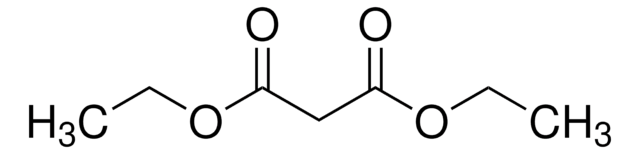
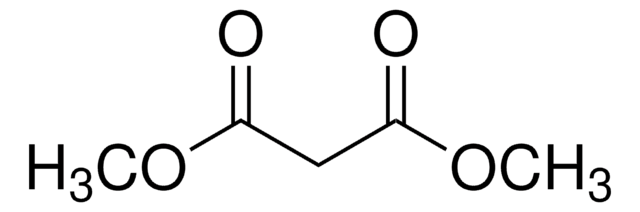
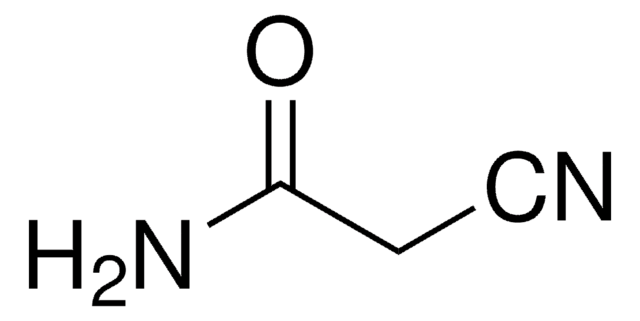
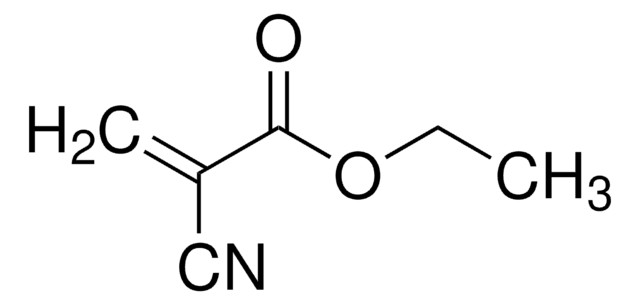
Produtos recomendados
densidade de vapor
3.9 (vs air)
Nível de qualidade
pressão de vapor
1 mmHg ( 67.8 °C)
Ensaio
≥98%
Formulário
liquid
índice de refração
n20/D 1.418 (lit.)
p.e.
208-210 °C (lit.)
pf
−22 °C (lit.)
densidade
1.063 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
CCOC(=O)CC#N
InChI
1S/C5H7NO2/c1-2-8-5(7)3-4-6/h2-3H2,1H3
chave InChI
ZIUSEGSNTOUIPT-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Ethyl cyanoacetate is widely used as a building block in organic synthesis to produce active pharmaceutical ingredients.
Aplicação
Embalagem
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
230.0 °F
Ponto de fulgor (°C)
110 °C
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| E18425-250G | 4061833602249 |
| E18425-250ML | |
| E18425-10L | |
| E18425-1KG | 4061833602232 |
| E18425-1L | |
| E18425-5G | 4061833602256 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica