About This Item
Fórmula linear:
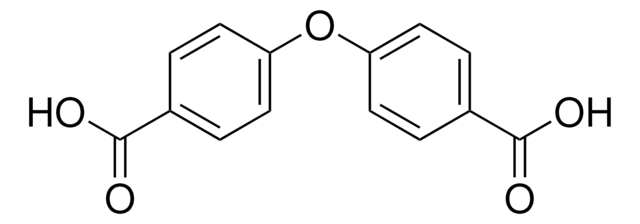
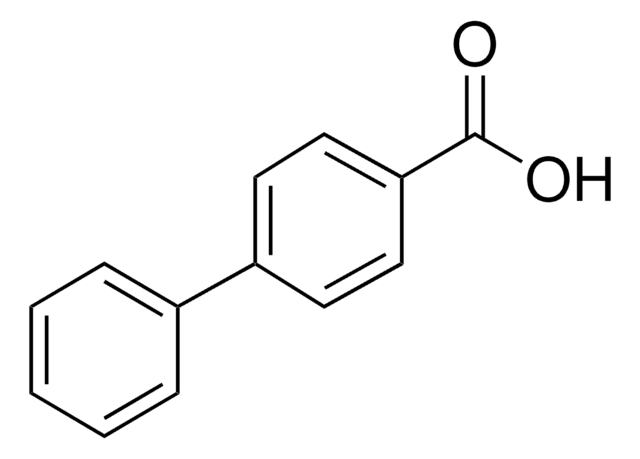
C6H5OC6H4CO2H
Número CAS:
Peso molecular:
214.22
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
pf
163-165 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
OC(=O)c1ccc(Oc2ccccc2)cc1
InChI
1S/C13H10O3/c14-13(15)10-6-8-12(9-7-10)16-11-4-2-1-3-5-11/h1-9H,(H,14,15)
chave InChI
RYAQFHLUEMJOMF-UHFFFAOYSA-N
Descrição geral
4-Phenoxybenzoic acid was converted to its corresponding amide by the soil bacterium Bacillus cereus Tim-r01.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
R Maruyama et al.
Bioscience, biotechnology, and biochemistry, 65(8), 1761-1765 (2001-10-02)
The soil bacterium Bacillus cereus Tim-r01 efficiently transformed polyaromatic carboxylic acids (PACA) such as 4-biphenylcarboxylic acid (4-BPCA), 4-biphenylacetic acid, and 4-phenoxybenzoic acid into their corresponding amides. The amidation activity was expressed at 37 degrees C (pH 7-8) in the presence
K H Engesser et al.
FEMS microbiology letters, 57(3), 317-321 (1990-06-01)
A bacterial strain, Pseudomonas sp. POB 310, was enriched with 4-carboxy biphenyl ether as sole source of carbon and energy. Resting cells of POB 310 co-oxidize a substrate analogue, 4-carboxybenzophenone, yielding 1,2-dihydro-1,2-dihydroxy-4-carboxy-benzophenone. The ether bond of 3- and 4-carboxy biphenyl
Hideki Moriyama et al.
Bioorganic & medicinal chemistry letters, 13(16), 2737-2740 (2003-07-23)
In order to investigate structure-activity relationships of azasugar series toward metalloproteinases, we synthesized and evaluated several azasugar-based compounds. As a result, it was found that 4-phenoxybenzene derivative 3 having 2R,3R,4R,5S-configurations exhibited most potent inhibitory activities against matrix metalloproteinase-1, -3 and
U Dehmel et al.
Archives of microbiology, 163(1), 35-41 (1995-01-01)
Pseudomonas pseudoalcaligenes strain POB310 degrades 3- and 4-carboxydiphenyl ether. The initial reaction involves an angular dioxygenation yielding an unstable hemiacetal that spontaneously decays to phenol and protocatechuate. We cloned a DNA fragment containing the gene encoding the initial dioxygenase from
Mikaela Nichkova et al.
Analytical chemistry, 77(21), 6864-6873 (2005-11-01)
Currently, detection in microarray bioanalysis is based mainly on the use of organic dyes. To overcome photobleaching and spectral overlaps we applied a new type of fluorophore, crystalline europium-doped gadolinium oxide (Eu:Gd2O3) nanoparticles, as labels in immunoassay microarrays. The Eu:Gd2O3
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica