C99000
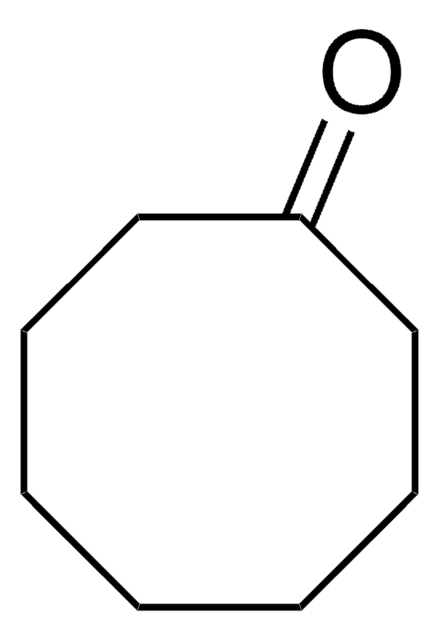
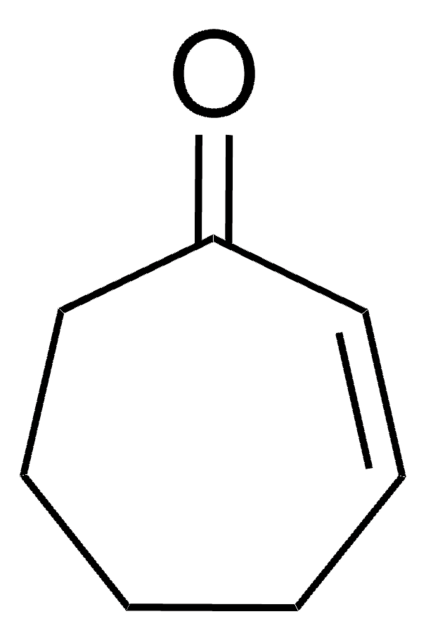
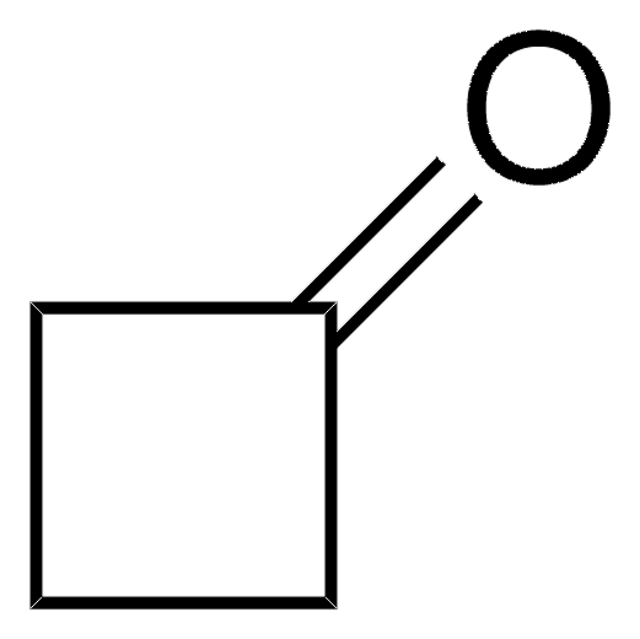
Cycloheptanone
99%
Sinônimo(s):
Ketocycloheptane, Ketoheptamethylene, Suberone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C7H12(=O)
Número CAS:
Peso molecular:
112.17
Beilstein:
969823
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
Formulário
liquid
índice de refração
n20/D 1.461 (lit.)
p.e.
179 °C (lit.)
densidade
0.951 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
O=C1CCCCCC1
InChI
1S/C7H12O/c8-7-5-3-1-2-4-6-7/h1-6H2
chave InChI
CGZZMOTZOONQIA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Flam. Liq. 3
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
131.0 °F
Ponto de fulgor (°C)
55 °C
Equipamento de proteção individual
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Brian T Sullivan et al.
Journal of chemical ecology, 47(1), 10-27 (2021-01-07)
We investigated geographic variation in the semiochemistry of major disturbance agents of western North American pine forests, Dendroctonus brevicomis Le Conte and Dendroctonus barberi Hopkins (Coleoptera: Curculionidae: Scolytinae), species separated by the Great Basin in the USA that until recently
R Singh et al.
Current medicinal chemistry. Anti-cancer agents, 3(6), 431-438 (2003-10-08)
A series of naturally occurring and synthetic novel oxapenam (4-oxa-1-azabicyclo[3.2.0] heptan-7-one) derivatives with their antitumor activity and the structure-activity relationship among this class of compounds is reported. Among the synthetic 4-oxa-1-azabicyclo[3.2.0]heptan-7-one having an ester, amide, ether derivatives of hydroxy group
Richmond Sarpong et al.
Journal of the American Chemical Society, 125(45), 13624-13625 (2003-11-06)
A set of mild processes for the conversion of vinyl cyclopropyl diazo ketones to highly functionalized cycloheptadienones and vinyl cyclopentenones by use of a target-inspired tandem Wolff/Cope rearrangement sequence is described. A divergent reaction course of the vinyl cyclopropyl diazo
M Juza
Journal of chromatography. A, 865(1-2), 35-49 (2000-02-16)
A binary test mixture consisting of cyclopentanone and cycloheptanone is used for the performance evaluation of a pilot-scale simulated moving bed unit. The involved adsorption equilibria and the kinetic behavior are discussed in detail. The results of the test runs
Redouane Beniazza et al.
The Journal of organic chemistry, 76(3), 791-799 (2011-01-13)
A short access to homocalystegine analogues silylated at C7 is described. The synthesis involves the desymmetrization of a (phenyldimethylsilyl)methylcycloheptatriene using osmium-mediated dihydroxylation, followed by the diol protection and a cycloaddition involving the remaining diene moiety and an acylnitroso reagent. Additions
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica