About This Item
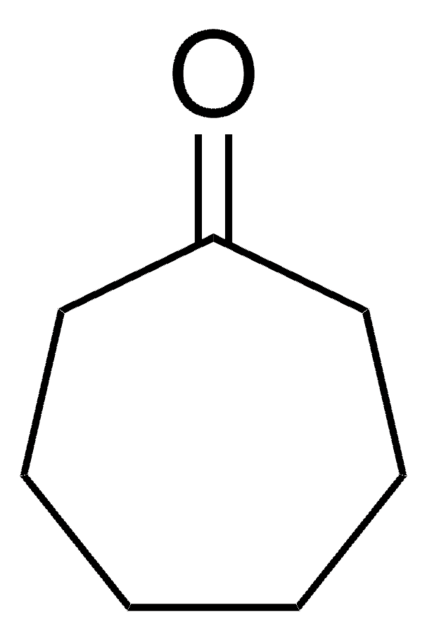
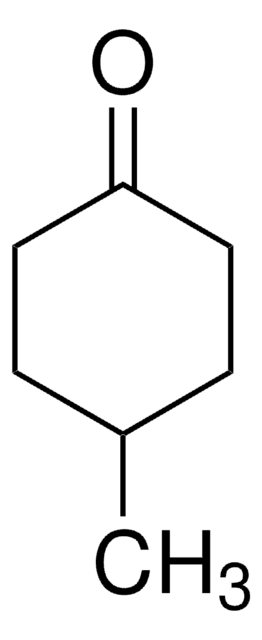
Fórmula linear:
C8H14(=O)
Número CAS:
Peso molecular:
126.20
Beilstein:
1280738
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
forma
crystals
pb
195-197 °C (lit.)
pf
32-41 °C (lit.)
densidade
0.958 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
O=C1CCCCCCC1
InChI
1S/C8H14O/c9-8-6-4-2-1-3-5-7-8/h1-7H2
chave InChI
IIRFCWANHMSDCG-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1 - Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
165.2 °F
Ponto de fulgor (°C)
74 °C
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Vishwakarma Singh et al.
The Journal of organic chemistry, 70(3), 973-981 (2005-01-29)
A new and efficient synthesis of a variety of highly embellished bicyclooctenones having an endo-vinyl moiety and their sigmatropic shifts in ground and excited states leading to a stereoselective route to substituted cis-decalins and diquinane frameworks have been described. Functionalized
M E Krafft et al.
The Journal of organic chemistry, 66(22), 7443-7448 (2001-10-30)
The total synthesis of asteriscanolide (1) has been achieved by taking advantage on an intermolecular Pauson-Khand cycloaddition and a ring-closing metathesis as key bond-forming transformations. The approach incorporates the cyclooctane stereogenic center prior to ring formation. Interestingly, the ring-closing metathesis
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 1898-1905 (1998-01-20)
Construction of the AB-ring system of the taxane framework via an A-ring annulation strategy was demonstrated by base-mediated intramolecular aldol reaction of (Z)-2,2-dimethyl-3-(1-methyl-2-oxopropylidene)cyclooctanone, affording the title compound, 1-hydroxy-8,11,11-trimethylbicyclo[5.3.1]undec-7-en-9-one. A cyclization precursor, the tetra-substituted (Z)-alkene, was prepared from the corresponding cyclooctanone
F E Harvey et al.
Brain research bulletin, 13(4), 541-547 (1984-10-01)
Female mice were reared in observation incubators from day 1 of life for three weeks. During that time they were continuously exposed to the odors of either cyclooctanone, adult male mouse urine or distilled water. The growth rate was temporarily
K Yamada et al.
Chemical & pharmaceutical bulletin, 45(12), 2113-2115 (1998-01-20)
Stereoselective syntheses of omega-(alpha-bromoketo) octanals and nonanal with oxygenated functions and formation of the corresponding eight-membered carbocyclic aldols by subsequent samarium(II)-mediated cyclization are demonstrated. Cyclooctenones deoxygenated at the C2 or C10 position in the taxane framework are prepared by dehydration
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica