C80857
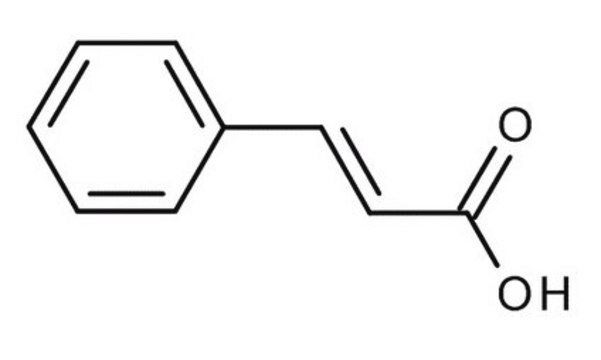
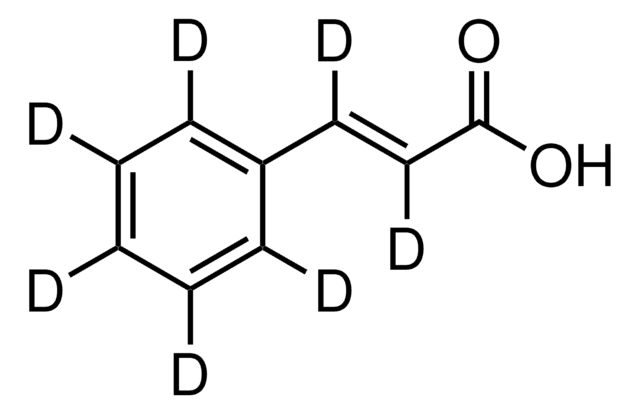
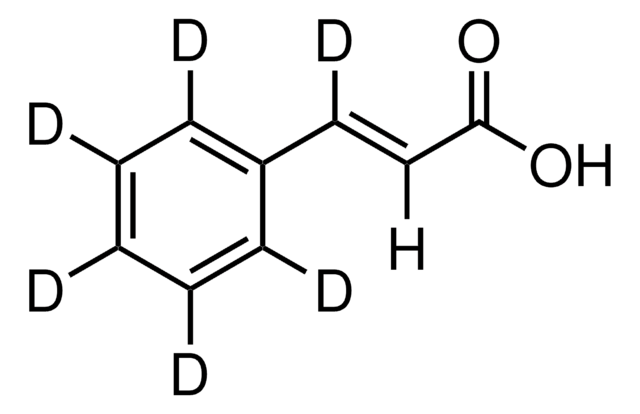
trans-Cinnamic acid
≥99%
Sinônimo(s):
trans-3-Phenylacrylic acid, Cinnamic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C6H5CH=CHCOOH
Número CAS:
Peso molecular:
148.16
Beilstein:
1905952
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
≥99%
Formulário
crystals
p.e.
300 °C (lit.)
pf
132-135 °C (lit.)
cadeia de caracteres SMILES
OC(=O)\C=C\c1ccccc1
InChI
1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+
chave InChI
WBYWAXJHAXSJNI-VOTSOKGWSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
trans-Cinnamic acid can be used in the synthesis of:
- A trans-cinnamic acid hydrazide derivative with potent anti-mycobacterial activity.
- Cinnamate glycerides via homogeneous esterification reaction.
- Styrene via biocatalytic decarboxylation by plant cell cultures.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
320.0 °F - closed cup
Ponto de fulgor (°C)
160 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives.
Carvalho SA, et al.
Bioorganic & Medicinal Chemistry, 18(2), 538-541 (2008)
Homogeneous catalytic esterification of glycerol with cinnamic and methoxycinnamic acids to cinnamate glycerides in solventless medium: Kinetic modeling.
Molinero L, et al.
Chemical Engineering Journal, 247(2), 174-182 (2014)
M Takemoto et al.
Chemical & pharmaceutical bulletin, 49(5), 639-641 (2001-06-01)
A novel method for producing styrenes from trans-cinnamic acids was developed. When trans-cinnamic acid was incubated with plant cell cultures at room temperature, styrene was obtained. 4-Hydroxy-3-methoxystyrene (2a), 3-nitrostyrene (2f) and furan (2g) were synthesized quantitatively.
Feng Yang et al.
Molecular pharmaceutics, 9(11), 3259-3265 (2012-09-27)
Owing to advantageous biochemical and pharmacological properties of human serum albumin (HSA), HSA-based drug carrier is playing an increasing role in the clinical setting. Since the IIA subdomain of HSA is a big hydrophobic cavity, we proposed that HSA delivers
Andrew M Lauer et al.
Organic letters, 14(19), 5138-5141 (2012-09-25)
A highly regioselective, Pd-catalyzed allylic fluorination of phosphorothioate esters is reported. This chemistry addresses several limitations of previously reported methods in which elimination and lack of reactivity were problematic. Preliminary mechanistic investigations reveal that these reactions are stereospecific and provide
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica