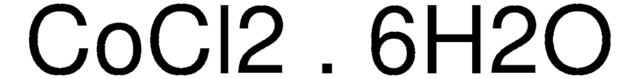769495
Cobalt(II) chloride hexahydrate
≥97%
Sinônimo(s):
Cobaltous chloride hexahydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
pressão de vapor
40 mmHg ( 0 °C)
Ensaio
≥97%
97.0-102.0% (KT)
Formulário
solid
traços de ânion
nitrate (NO3-): ≤0.01%
sulfate (SO42-): ≤0.007%
traços de cátion
Fe: ≤0.005%
Ni: ≤0.15%
Pb: ≤0.002%
Zn: ≤0.05%
cadeia de caracteres SMILES
O.O.O.O.O.O.Cl[Co]Cl
InChI
1S/2ClH.Co.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2
chave InChI
GFHNAMRJFCEERV-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- An additive to the electron transport layer (ETL) in perovskite solar cells to improve their performance, particularly by reducing energy losses and increasing the open-circuit voltage.
- A cobalt source for doping ZnO nanostructures. The incorporation of cobalt ions into the ZnO matrix is crucial for modifying its electronic and optical properties.
- A precursor to modify cobalt metal-organic framework (Co-MOF) derived carbon microspheres for application as anode materials in lithium-ion batteries.
Nota de análise
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica