529249
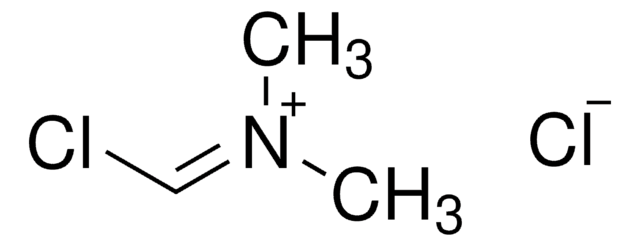
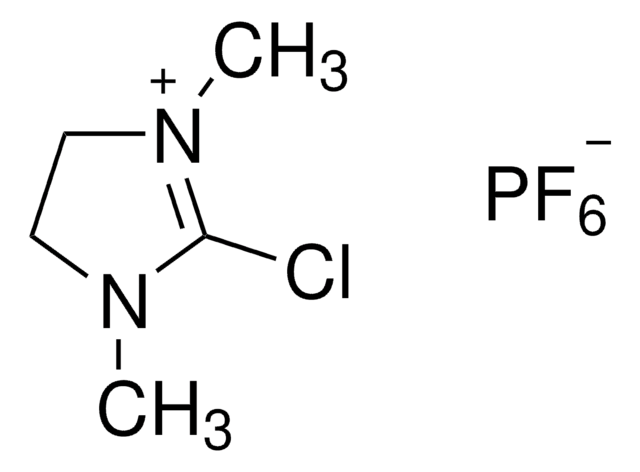
2-Chloro-1,3-dimethylimidazolinium chloride
for peptide synthesis
Sinônimo(s):
2-Chloro-4,5-dihydro-1,3-dimethyl-1H-imidazolium chloride, DMC
About This Item
Produtos recomendados
Nome do produto
2-Chloro-1,3-dimethylimidazolinium chloride,
Formulário
crystalline
Nível de qualidade
adequação da reação
reaction type: Coupling Reactions
pf
133-140 °C (lit.)
aplicação(ões)
peptide synthesis
grupo funcional
chloro
cadeia de caracteres SMILES
[Cl-].CN1CC[N+](C)=C1Cl
InChI
1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1
chave InChI
AEBBXVHGVADBHA-UHFFFAOYSA-M
Aplicação
Tagged glucose as an intermediate in the synthesis of branched oligosaccharides
Fluorescent chemosensors
1,2-Diamines as inhibitors of co-activator associated arginine methyltransferase 1
Allosteric glucokinase activators
Reactant for synthesis of:
Organic azides from primary amines
Reagent for aza-Henry reactions
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica