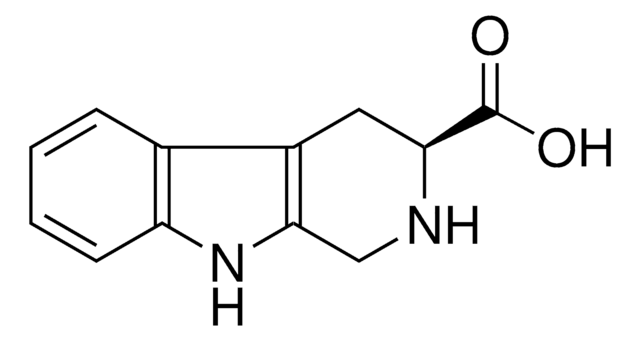
300764
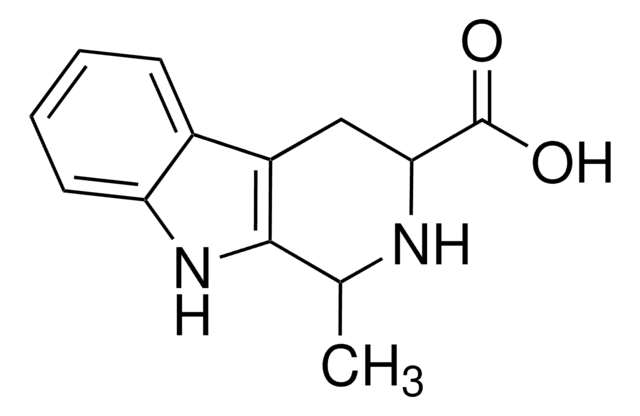
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole
98%
Sinônimo(s):
Noreleagnine, THBC, Tetrahydronorharman, Tryptoline
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C11H12N2
Número CAS:
Peso molecular:
172.23
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
liquid
pf
206-208 °C (lit.)
cadeia de caracteres SMILES
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
chave InChI
CFTOTSJVQRFXOF-UHFFFAOYSA-N
Informações sobre genes
rat ... Htr2a(29595) , Htr2c(25187)
Categorias relacionadas
Descrição geral
Ozonolysis of the enamine bond of 1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole derivatives was studied.
Aplicação
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole was employed in alkaloid synthesis and in studies on neurodegenerative diseases.
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Karolina Pulka
Current opinion in drug discovery & development, 13(6), 669-684 (2010-11-10)
The synthesis of biologically active heterocyclic scaffolds is one of the significant challenges of modern synthetic chemistry. The Pictet-Spengler (PS) reaction, known for approximately a century, remains a particularly popular cyclization method. This review describes recent applications of the PS
Tetrahedron, 48, 9735-9735 (1992)
Fangrui Wu et al.
Journal of the American Chemical Society, 134(3), 1498-1500 (2012-01-11)
The Pictet-Spenglerase strictosidine synthase (STR1) has been recognized as a key enzyme in the biosynthesis of some 2000 indole alkaloids in plants, some with high therapeutic value. In this study, a novel function of STR1 has been detected which allows
Kaushik Chanda et al.
Molecular diversity, 15(2), 569-581 (2010-10-12)
The Pictet-Spengler reaction, using polyethylene glycol immobilized tryptophan ester with a variety of ketones, was achieved by refluxing condition in acidic chloroform. The linear as well as cyclic ketones were employed. All the ketones were reacted within 6-8 h to
I M McDonald et al.
Journal of medicinal chemistry, 43(19), 3518-3529 (2000-09-23)
A novel series of nonpeptide CCK(2) receptor antagonists has been prepared, in which 2,7-dioxo-2,3,4,5,6,7-hexahydro-1H-benzo[h][1, 4]diazonine (5) was used as a chemical template. This uncommon ring system was obtained in a highly substituted form and in high yield by ozonolysis of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
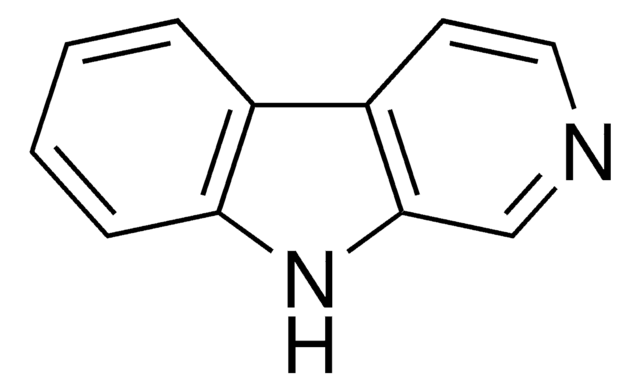
![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)