295035
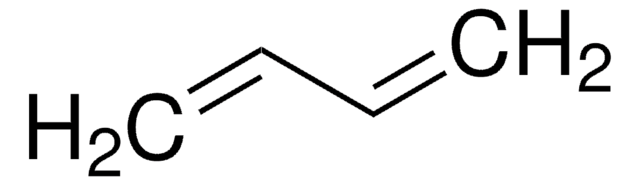
1,3-Butadiene
≥99%
Sinônimo(s):
Bivinyl, Vinylethylene, alpha,gamma-Butadiene
About This Item
Produtos recomendados
densidade de vapor
1.9 (15 °C, vs air)
Nível de qualidade
pressão de vapor
1863 mmHg ( 21 °C)
Ensaio
≥99%
temperatura de autoignição
788 °F
contém
p-tert-butylcatechol as inhibitor
Lim. expl.
12 %
p.e.
−4.5 °C (lit.)
pf
−109 °C (lit.)
solubilidade
water: soluble 0.5 g/L at 20 °C
densidade
0.62 g/mL at 20 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
C=CC=C
InChI
1S/C4H6/c1-3-4-2/h3-4H,1-2H2
chave InChI
KAKZBPTYRLMSJV-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
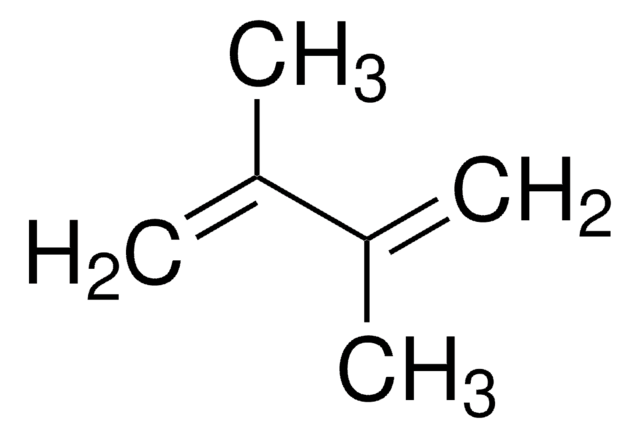
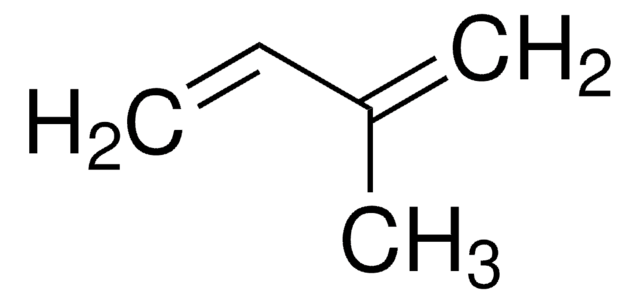
It may be used in the synthesis of the following:
- 1-Silyl-substituted 1,3-butadienes, by [RuHCl(CO)(PCy3)2]-catalyzed silylative coupling of terminal (E)-1,3-dienes with vinylsilanes.
- Synthetic rubber and thermoplastic resins.
- Disilylated dimers by reacting with chlorosilanes.
- Octa-2,7-dien-1-ol via palladium catalyzed-hydrodimerization.
Ações bioquímicas/fisiológicas
Embalagem
Compatible with the following:
Informações legais
espiga da mangueira
geralmente comprado junto com este produto
recomendado
regulador
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Gas 1A - Muta. 1B - Press. Gas Liquefied gas
Código de classe de armazenamento
2A - Gases
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
-104.8 °F - closed cup
Ponto de fulgor (°C)
-76 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Protocolos
2-Butene; 2-Methylbutane; 1,3-Butadiene; Propyne
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica