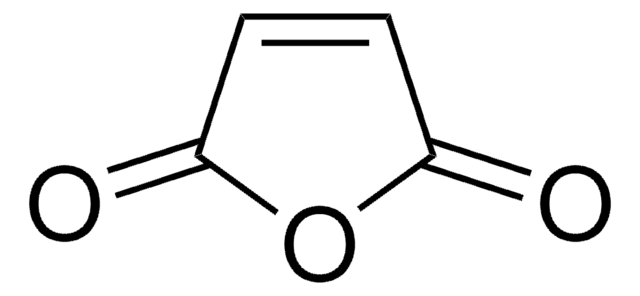
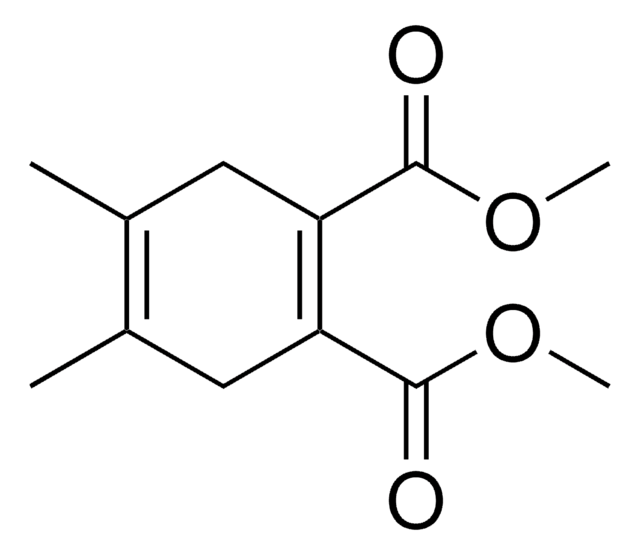
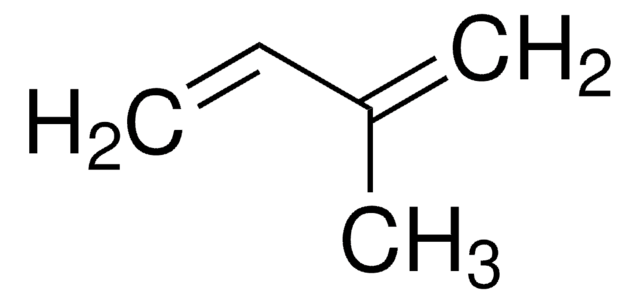
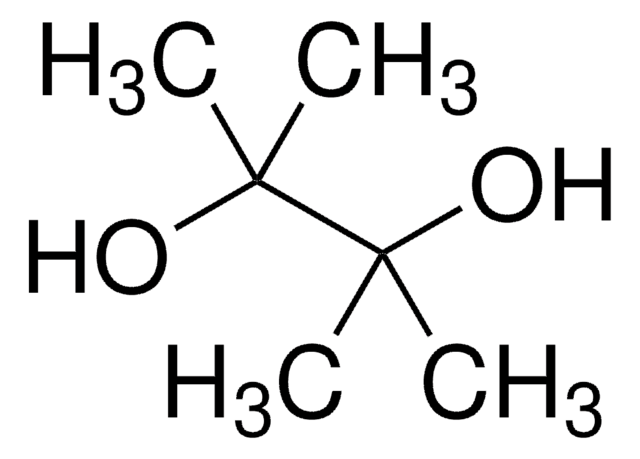
145491
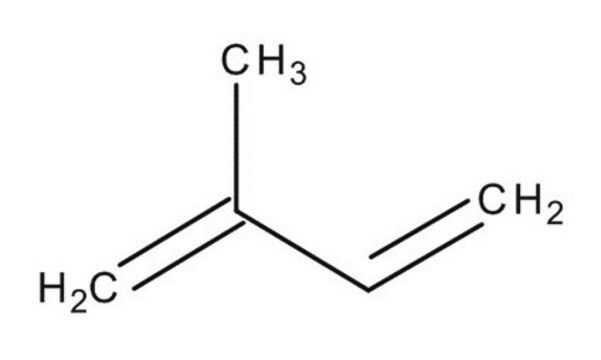
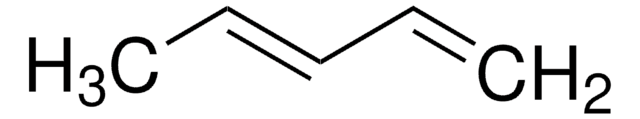
2,3-Dimethyl-1,3-butadiene
98%, contains 100 ppm BHT as stabilizer
Sinônimo(s):
2,3-Dimethylbuta-1,2-diene, 2,3-Dimethylenebutane, Biisopropenyl, Diisopropenyl
About This Item
Produtos recomendados
pressão de vapor
269 mmHg ( 37.7 °C)
Nível de qualidade
Ensaio
98%
forma
liquid
contém
100 ppm BHT as stabilizer
índice de refração
n20/D 1.438 (lit.)
pb
68-69 °C (lit.)
pf
−76 °C (lit.)
densidade
0.726 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
chave InChI
SDJHPPZKZZWAKF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
It may be used in the following processes:
- Preparation of 1,3,6-triene derivatives of corresponding 1-aryl-substituted 1,3-dienes by 1,4-hydrobutadienylation in the presence of cobalt catalyst.
- Synthesis of 6-aryl(hetaryl)-3,4-dimethyl-1-nitro-1-cyano-3-cyclohexenes by reacting with gem-cyanonitroethenes.
- As a halogen trap during the study of the photolysis reaction of dibromo adduct of 2,5-diphenyltellurophene.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
30.2 °F - closed cup
Ponto de fulgor (°C)
-1 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica