274984
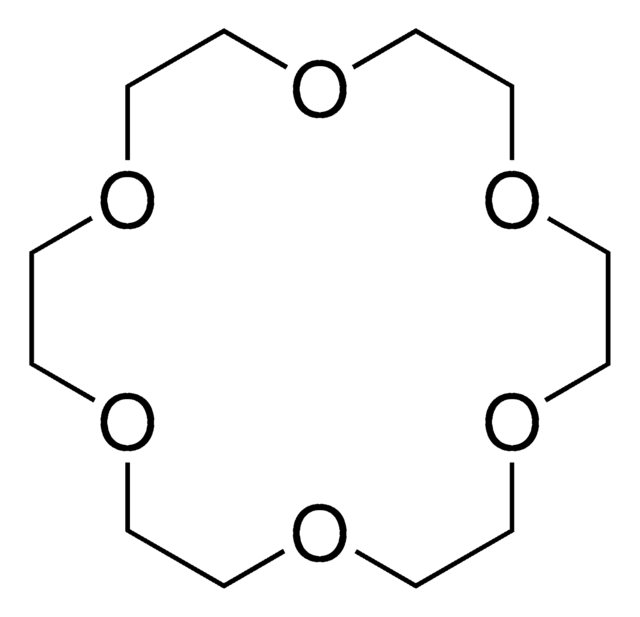
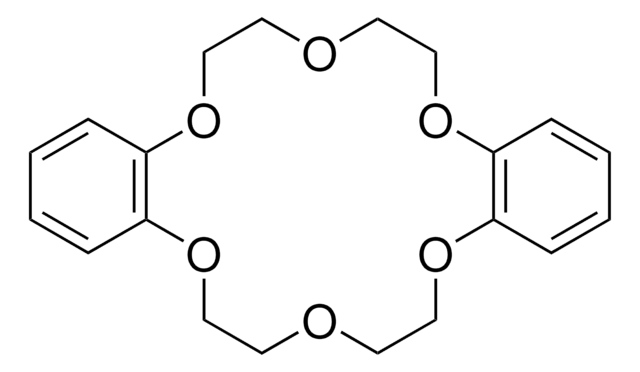
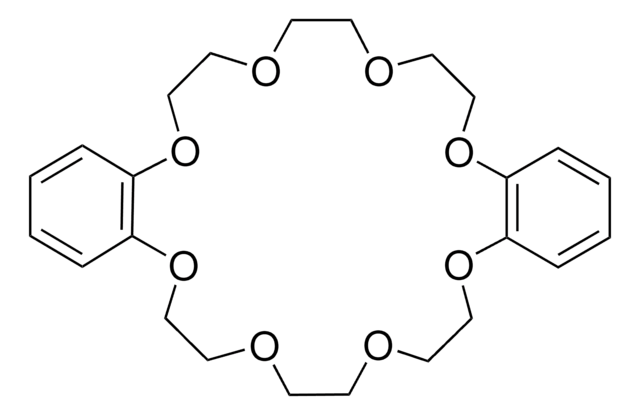
18-Crown-6
≥99.0%
Sinônimo(s):
1,4,7,10,13,16-Hexaoxacyclooctadecane
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C12H24O6
Número CAS:
Peso molecular:
264.32
Beilstein:
1619616
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
≥99.0%
Formulário
solid
pf
42-45 °C (lit.)
grupo funcional
ether
cadeia de caracteres SMILES
O1CCOCCOCCOCCOCCOCC1
InChI
1S/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
chave InChI
XEZNGIUYQVAUSS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
18-Crown-6 is a macrocyclic polyether used to synthesize ionic liquid based crown-ether coordination compounds.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Aplicação
18-Crown-6 can be used as a catalyst for:
- N-alkylation of heterocyclic compounds in the presence of tert-butoxide base.
- Allylation of aldehydes to corresponding homoallylic alcohols using potassium allyltrifluoroborate.
- Preparation of N-propargylpyrrole by the reaction of pyrrole with potassium hydroxide.
- Polymerization of methacrylic esters and hindered alkyl acrylates.
- Chemoselective reduction of fused tetrazoles with NaBH4 and potassium hydroxide.
18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Can be useful as phase-transfer catalysts.
Outras notas
Macrocyclic polyethers with repeating (-CH2CH2O) units. The compounds are ionophoric (form stable complexes with cations).
produto relacionado
Nº do produto
Descrição
Preços
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Magnetic blocking at 10 K and a dipolar-mediated avalanche in salts of the bis (?8-cyclooctatetraenide) complex [Er (COT) 2]?.
Meihaus K R, et al.
Journal of the American Chemical Society, 135(47), 17952-17957 (2013)
18?Crown?6.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Completing the series of +2 ions for the lanthanide elements: synthesis of molecular complexes of Pr2+, Gd2+, Tb2+, and Lu2+.
MacDonald M R, et al.
Journal of the American Chemical Society, 135(26), 9857-9868 (2013)
18?Crown?6.
Organic Syntheses (2003)
Improved synthesis and efficient chemoselective reduction of fused tetrazoles under phase-transfer conditions
Desai ND and Shah RD
Synthesis, 2006(19), 3275-3279 (2006)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica