426288
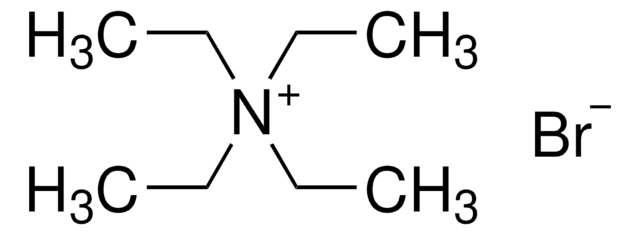
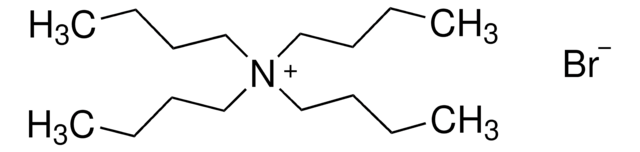
Tetrabutylammonium bromide
ACS reagent, ≥98.0%
Sinônimo(s):
N,N,N-tributyl-1-butanaminium bromide, TBAB, TBABr, tetra-n-butylammonium bromide
About This Item
Produtos recomendados
grau
ACS reagent
Nível de qualidade
Ensaio
≥98.0%
Formulário
solid
características do produto alternativo mais ecológico
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezas
≤0.5% tributylamine hydrobromide
≤0.5% tributylamine
pf
102-106 °C (lit.)
grupo funcional
amine
categoria alternativa mais ecológica
cadeia de caracteres SMILES
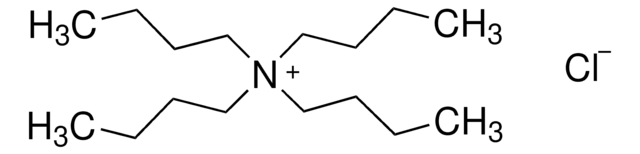
[Br-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.BrH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
chave InChI
JRMUNVKIHCOMHV-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Synthesis of (2S)-5-(3-phenyl-2-phthalimidylpropanoylamino)isophthalic acid.
- Synthesis of alkyl-substituted pyrroles in the absence of catalyst and organic solvent.
- Synthesis of dithioacetals from acetals by transthioacetalisation in a solvent free environment.
- Synthesis of polyamides (PAs) by the polymerization of terephthalic acid and diisocyanates.
- Catalyze the addition of thiols to conjugated alkenes.
- Dehydrochlorination of poly(vinyl chloride).
Process for Producing Halogenated Heteroaryl Compounds
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![4-[4-(Dimethylamino)styryl]pyridine 95%](/deepweb/assets/sigmaaldrich/product/structures/225/605/ad18cc93-9d43-467b-8618-105948f9692b/640/ad18cc93-9d43-467b-8618-105948f9692b.png)

![trans-4-[4-(Dimethylamino)styryl]-1-methylpyridinium iodide Dye content 98 %](/deepweb/assets/sigmaaldrich/product/structures/416/722/5d59b6c3-5f2d-4396-a721-5cb82ba7038c/640/5d59b6c3-5f2d-4396-a721-5cb82ba7038c.png)