264431
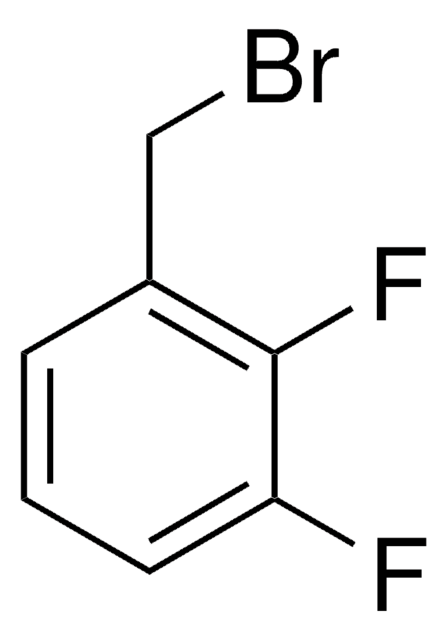
2,6-Difluorobenzyl bromide
97%
Sinônimo(s):
α-Bromo-2,6-difluorotoluene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
F2C6H3CH2Br
Número CAS:
Peso molecular:
207.02
Beilstein:
2083943
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
forma
solid
pf
52-55 °C (lit.)
grupo funcional
bromo
cadeia de caracteres SMILES
Fc1cccc(F)c1CBr
InChI
1S/C7H5BrF2/c8-4-5-6(9)2-1-3-7(5)10/h1-3H,4H2
chave InChI
LSXJPJGBWSZHTM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
2,6-Difluorobenzyl bromide has been used:
- as reagent in alkylation of the quinazoline-2-thioxo-4-one
- in the synthesis of 1,3,5-triazine-2,4,6-triones
- in the preparation of new classes of inhibitors of bovine viral diarrhea virus (as a surrogate virus for hepatitis C virus)
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Dam. 1 - Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Zhiqiang Guo et al.
Bioorganic & medicinal chemistry letters, 15(3), 693-698 (2005-01-25)
A convenient one-pot synthetic route was developed for the preparation of asymmetric 1,3-dialkyl-1,3,5-triazine-2,4,6-triones from readily available alkyl- or aryl-isocyanates, primary amines and N-chlorocarbonyl isocyanate in excellent yields. Subsequent alkylation with N-protected amino alcohols afforded the desired 1,3,5-triazine-2,4,6-triones in good yields.
Gerhard Puerstinger et al.
Bioorganic & medicinal chemistry letters, 16(20), 5345-5349 (2006-08-12)
A novel class of inhibitors of pestiviruses (5-substituted 2-phenyl-5H-imidazo[4,5-c]pyridines) is described. Modification of the substituent in position 5 resulted in analogues with high activity (EC(50)<100nM) and selectivity (SI>1000) against the pestivirus BVDV (bovine viral diarrhea virus).
Efficient solid-phase synthesis of quinazoline-2-thioxo-4-ones with SynPhase? lanterns.
Makino S, et al.
Tetrahedron Letters, 41(43), 8333-8337 (2000)
Gwang-Noh Ahn et al.
Lab on a chip, 19(20), 3535-3542 (2019-09-27)
Microreactors are emerging as an efficient, sustainable synthetic tool compared to conventional batch reactors. Here, we present a new numbering-up metal microreactor by integrating a flow distributor and a copper catalytic module for high productivity of a commercial synthetic drug.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica