230669
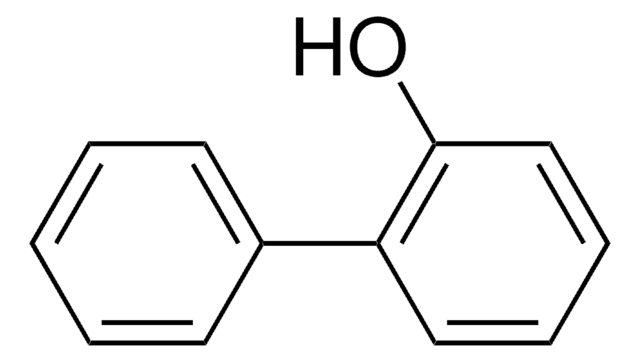
4-Phenoxyphenol
99%
Sinônimo(s):
Hydroquinone monophenyl ether
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C6H5OC6H4OH
Número CAS:
Peso molecular:
186.21
Beilstein:
2047182
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
Formulário
solid
pf
80-84 °C (lit.)
grupo funcional
phenoxy
cadeia de caracteres SMILES
Oc1ccc(Oc2ccccc2)cc1
InChI
1S/C12H10O2/c13-10-6-8-12(9-7-10)14-11-4-2-1-3-5-11/h1-9,13H
chave InChI
ZSBDGXGICLIJGD-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
338.0 °F - closed cup
Ponto de fulgor (°C)
170 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Victoria B F Custodis et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(36), 8658-8668 (2017-04-08)
One of the key challenges in renewable chemical production is the conversion of lignin, especially by fast pyrolysis. The complexity of the lignin pyrolysis process has hindered the elucidation of the mechanism, inhibiting further industrial implementation. By combining pyrolysis of
Kyoungseon Min et al.
Biotechnology for biofuels, 10, 212-212 (2017-09-16)
In the biorefinery utilizing lignocellulosic biomasses, lignin decomposition to value-added phenolic derivatives is a key issue, and recently biocatalytic delignification is emerging owing to its superior selectivity, low energy consumption, and unparalleled sustainability. However, besides heme-containing peroxidases and laccases, information
Xiaolu Jiang et al.
Bioorganic & medicinal chemistry letters, 18(24), 6549-6552 (2008-10-28)
The synthesis and biological evaluation of a series of diphenyl ether derivatives were described. The compounds can either activate or inhibit the aminopeptidase activity of leukotriene A(4) hydrolase, while at the same time do not influence the hydrolase activity. Further
Cynthia D Selassie et al.
Journal of medicinal chemistry, 48(23), 7234-7242 (2005-11-11)
In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop the following
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica