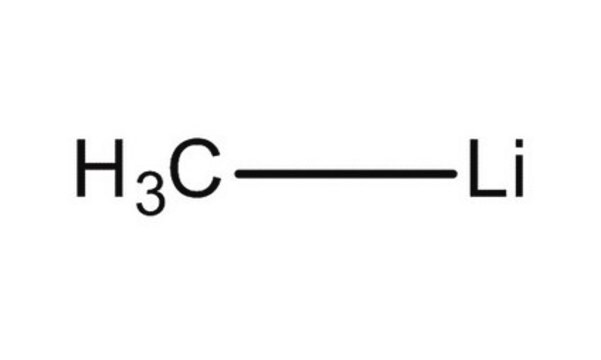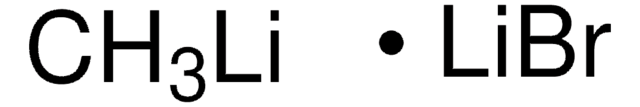197343
Methyllithium solution
1.6 M in diethyl ether
Sinônimo(s):
Lithium methanide, MeLi
About This Item
Produtos recomendados
densidade de vapor
3 (vs air)
Nível de qualidade
forma
liquid
composição
halide, ~0.05 M
concentração
1.6 M in diethyl ether
densidade
0.732 g/mL at 25 °C
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[Li]C
InChI
1S/CH3.Li/h1H3;
chave InChI
DVSDBMFJEQPWNO-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Embalagem
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Perigos de suplementos
Código de classe de armazenamento
4.2 - Pyrophoric and self-heating hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
1.4 °F - closed cup
Ponto de fulgor (°C)
-17 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica