154180
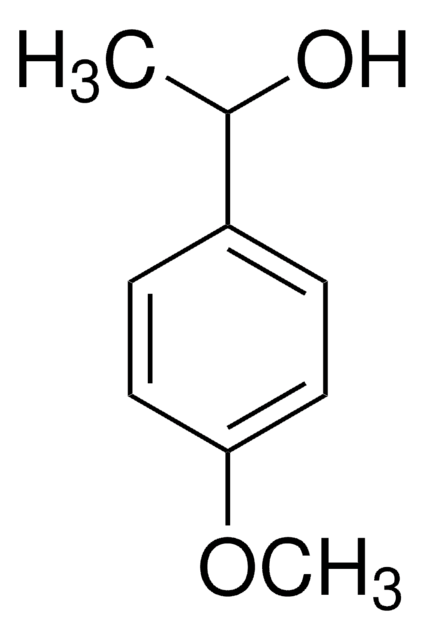
4-Methoxyphenethyl alcohol
99%
Sinônimo(s):
2-(4-Methoxyphenyl)ethanol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3OC6H4CH2CH2OH
Número CAS:
Peso molecular:
152.19
Beilstein:
2043563
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
Formulário
solid
p.e.
334-336 °C (lit.)
pf
26-28 °C (lit.)
grupo funcional
hydroxyl
cadeia de caracteres SMILES
COc1ccc(CCO)cc1
InChI
1S/C9H12O2/c1-11-9-4-2-8(3-5-9)6-7-10/h2-5,10H,6-7H2,1H3
chave InChI
AUWDOZOUJWEPBA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
(R)-1-(4-methoxyphenyl)ethanol {(R)-MOPE, 4-Methoxyphenethyl alcohol, 1-(4-methoxyphenyl)ethanol } is formed during the biocatalytic anti-Prelog enantioselective reduction of 4-methoxyacetophenone (MOAP) using immobilized Trigonopsis variabilis AS2.
Aplicação
4-Methoxyphenethyl alcohol was used as an internal standard in the fluorous biphasic catalysis reaction.
4-Methoxyphenethyl alcohol was used in the preparation of 4-(2-iodoethyl)phenol, by refluxing it with 47% hydriodic acid. It may be used in the preparation of (2R*,4R*)-1-n-butyl-2-methyl-4-(2-oxopyrrolidin-1-yl)-6-methoxy-1,2,3,4-tetrahydroquinoline and (2R*,4S*)-1-n-butyl-2-methyl-4-(2-oxopyrrolidin-1-yl)-6-methoxy-1,2,3,4- tetrahydroquinoline.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Biocatalytic anti-Prelog stereoselective reduction of 4'-methoxyacetophenone to (R)-1-(4-methoxyphenyl) ethanol with immobilized Trigonopsis variabilis AS2. 1611 cells using an ionic liquid-containing medium.
Lou W-Y, et al.
Green Chemistry, 11(9), 1377-1384 (2009)
Synthesis of 5-(ω-sulfhydrylalkyl) salicylaldehydes as precursors for the preparation of alkanethiol-modified metal salens.
Ji C and Peters DG
Tetrahedron Letters, 42(35), 6065-6067 (2001)
An asymmetric catalytic carbon? carbon bond formation in a fluorous biphasic system based on perfluoroalkyl-BINOL.
Tian Y and Chan KS.
Tetrahedron Letters, 41(45), 8813-8816 (2000)
Reactions of Azides with Electrophiles: New Methods for the Generation of Cationic 2-Azabutadienes. Synthesis of 1, 2, 3, 4-Tetrahydroquinolines and 1, 2-Dihydroquinolines via a Hetero Diels-Alder Reaction.
Pearson WH and Fang WK
Israel J. Chem., 37(1), 39-46 (1997)
Min Kyung Song et al.
Journal of agricultural and food chemistry, 67(7), 2028-2035 (2019-01-31)
Caffeic acid phenethyl ester (CAPE) is an ester of a hydroxycinnamic acid (phenylpropanoid) and a phenylethanoid (2-phenylethanol; 2-PE), which has long been used in traditional medicine. Here, we synthesized 54 hydroxycinnamic acid-phenylethanoid esters by feeding 64 combinations of hydroxycinnamic acids
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica