140414
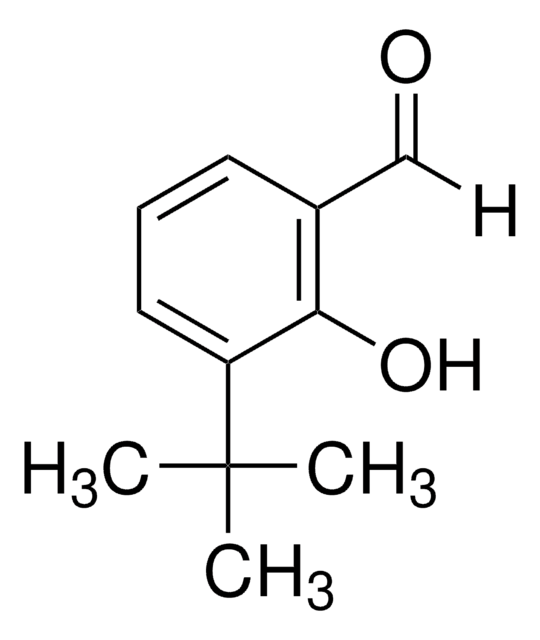
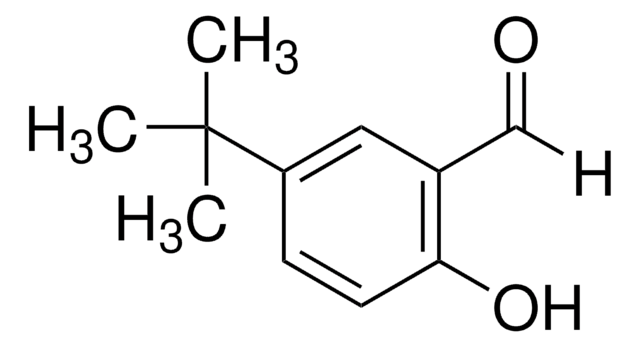
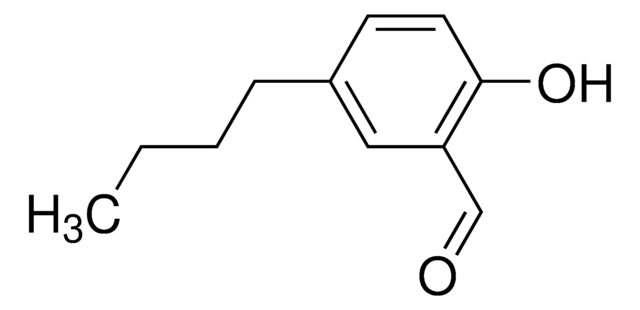
3,5-Di-tert-butyl-2-hydroxybenzaldehyde
99%
Sinônimo(s):
3,5-Di-tert-butylsalicylaldehyde
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HOC6H2[C(CH3)3]2CHO
Número CAS:
Peso molecular:
234.33
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
Formulário
powder or crystals
pf
59-61 °C (lit.)
grupo funcional
aldehyde
cadeia de caracteres SMILES
CC(C)(C)c1cc(C=O)c(O)c(c1)C(C)(C)C
InChI
1S/C15H22O2/c1-14(2,3)11-7-10(9-16)13(17)12(8-11)15(4,5)6/h7-9,17H,1-6H3
chave InChI
RRIQVLZDOZPJTH-UHFFFAOYSA-N
Descrição geral
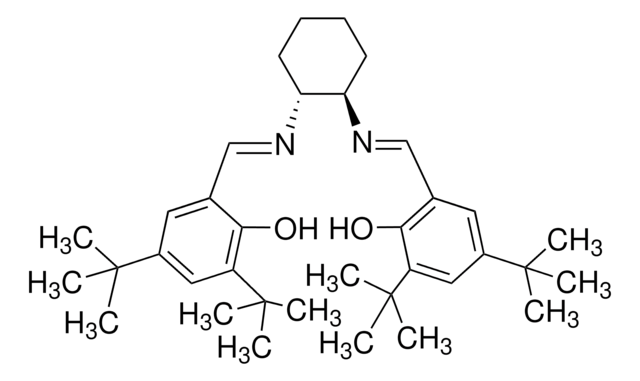
3,5-Di-tert-butyl-2-hydroxybenzaldehyde undergoes condensation reaction with
- methyl-2-{N-(2′-aminoethane)}-amino-1-cyclopentenedithiocarboxylate to yield Schiff base ligand
- N,N-diethyl-2-methyl-1,4-phenylenediamine during the synthesis of copper(II) and cobalt(II) complexes of salicylaldimine
Aplicação
3,5-Di-tert-butyl-2-hydroxybenzaldehyde was used in the synthesis of
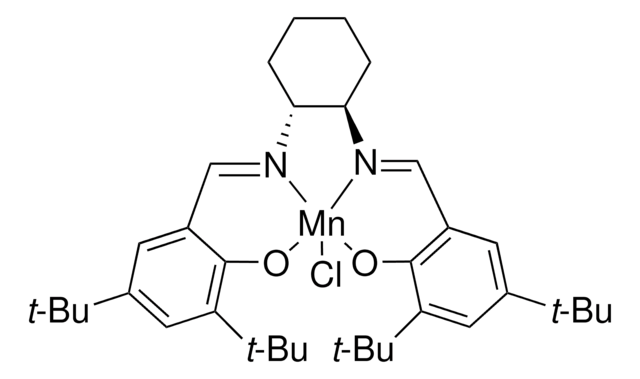
- Mn(III)-salen complex and its diamino precursor 5,6-diamino-5,6-dideoxy-1,2-O-isopropylidene-3-O-methyl-β-L-idofuranose
- chiral Schiff base ligand for an enantioselective copper-catalyzed addition of phenylacetylene to imines
- chiral oxazolidine ligand for the enantioselective addition of diethylzinc to aldehydes
- tin Schiff base complexes with histidine analogues
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Tetrahedron Asymmetry, 18, 377-377 (2007)
Chunshuang Liang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 183, 267-274 (2017-04-30)
A Schiff-base, (2,4-di-tert-butyl-6-((2-hydroxyphenyl-imino)-methyl)phenol) (L), has been improved to function as a simultaneous multi-ion probe in different optical channel. The probe changes from colorless to orangish upon being deprotonated by F
Mild and efficient synthesis of 5, 6-diamino-5, 6-dideoxy-1, 2-O-isopropylidene-3- O -methyl-?-l-idofuranose: precursor of the first carbohydrate-derived chiral Mn (III)-salen complex.
Yan S and Klemm D.
Tetrahedron, 58(50), 10065-10071 (2002)
Co (II) and Cu (II) Schiff base complexes of bis (N-(4-diethylamino-2-methylphenyl)-3, 5-di-tert-butylsalicylaldimine): Electrochemical and X-ray structural study.
Ulusoy M, et al.
Structural Chemistry, 19(5), 749-755 (2008)
Ariadna Garza-Ortiz et al.
Bioinorganic chemistry and applications, 2013, 502713-502713 (2013-07-19)
Five novel tin Schiff base complexes with histidine analogues (derived from the condensation reaction between L-histidine and 3,5-di-tert-butyl-2-hydroxybenzaldehyde) have been synthesized and characterized. Characterization has been completed by IR and high-resolution mass spectroscopy, 1D and 2D solution NMR ((1)H, (13)C and
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica