111279
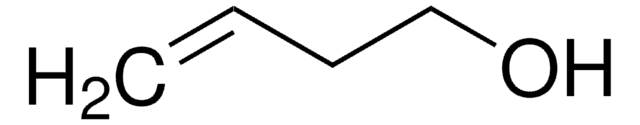
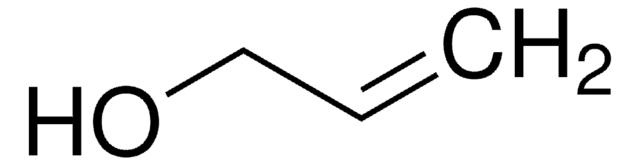
4-Penten-1-ol
99%
Sinônimo(s):
2-Allylethyl alcohol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH2=CH(CH2)3OH
Número CAS:
Peso molecular:
86.13
Beilstein:
1560163
Número CE:
Número MDL:
Código UNSPSC:
12352100
eCl@ss:
39020310
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
índice de refração
n20/D 1.429 (lit.)
p.e.
134-137 °C (lit.)
densidade
0.834 g/mL at 25 °C (lit.)
grupo funcional
allyl
hydroxyl
cadeia de caracteres SMILES
OCCCC=C
InChI
1S/C5H10O/c1-2-3-4-5-6/h2,6H,1,3-5H2
chave InChI
LQAVWYMTUMSFBE-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
4-penten-1-ol forms ester bond at the C terminus of the linear peptide in solution with HATU as coupling agent.
Aplicação
4-Penten-1-ol can be used as a reactant to prepare sulfamate ester by reacting with chlorosulfonyl isocycanate (142662).The derived ester undergoes an enantioselective intramolecular azridination reaction in the presence of Cu catalyst. 4-Penten-1-ol can also be used to study the epoxidation of olefins with oxo-diperoxo tungstate(VI) complex as catalyst and bicarbonate as co-catalyst.
Palavra indicadora
Warning
Frases de perigo
Classificações de perigo
Flam. Liq. 3
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
118.4 °F - closed cup
Ponto de fulgor (°C)
48 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Enantioselective Intramolecular Copper-Catalyzed Aziridination of Sulfamates
Audrey Esteoule.et al.
Synthesis, 1251-1251 (2007)
Highly efficient epoxidation method of olefins with hydrogen peroxide as terminal oxidant, bicarbonate as a co-catalyst and oxodiperoxo molybdenum(VI) complex as catalyst.
Maiti SK, et al.
New. J. Chem., 30(3), 479-489 (2006)
Stefania Terracciano et al.
Bioorganic & medicinal chemistry, 16(13), 6580-6588 (2008-05-30)
In the recent years, we focused our attention on the cyclodepsipeptide Jaspamide 1, an interesting marine metabolite, possessing a potent inhibitory activity against breast and prostate cancer, as a consequence of its ability to disrupt actin cytoskeleton dynamics. Although its
Marina D Rvovic et al.
Journal of molecular modeling, 17(6), 1251-1257 (2010-08-17)
The mechanism of phenylselenoetherification of pent-4-en-1-ol using some bases (pyridine, triethylamine, quinoline, 2,2'-bipyridine) as catalyst was examined through studies of kinetics of the cyclization, by UV-VIS spectrophotometry. It was demonstrated that the intramolecular cyclization is facilitated in the presence of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica