T2327
Trypsin inhibitor
lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
Synonym(s):
Kunitz Inhibitor
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
Product Name
Trypsin Inhibitor from Glycine max (soybean), BioUltra, lyophilized powder, ≥95% (Kunitz inhibitor, SDS-PAGE)
biological source
Glycine max (soybean)
Quality Level
product line
BioUltra
assay
≥95% (Kunitz inhibitor, SDS-PAGE)
form
lyophilized powder
storage temp.
2-8°C
Related Categories
General description
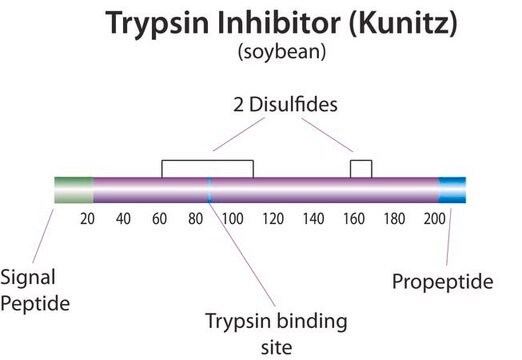
Trypsin Inhibitor from Glycine max (soybean) also known as Kunitz trypsin inhibitor is a 21 kDa protein with a single trypsin binding reactive site.
Application
Trypsin Inhibitor from Glycine max (soybean) has been used:
- as a standard protein to measure the amount of endogenous trypsin inhibitor present in midgut lysate (M1) of Riptortus pedestris
- as a standard to compare the trypsin inhibitory activity of the purified protein
- to monitor the trypsin inhibitory activity by fractionating in MonoS cation exchange chromatography
- as an trypsin inhibitor
Biochem/physiol Actions
Soybean trypsin inhibitor inhibits trypsin and to a lesser extent chymotrypsin and plasmin. It forms a 1:1 stoichiometric complex with trypsin. Upon formation of this complex, trypsin may cleave a single arginine-isoleucine bond in the inhibitor. Dissociation of this complex may yield the modified form or the native inhibitor. At the optimal pH for trypsin binding (pH 8.0), the association constant is ≥ 10x108.
Trypsin Inhibitor from Glycine max (soybean) binds with the active site of trypsin enzyme, in a competitive inhibition manner.
Unit Definition
One trypsin unit will produce a ΔA253 of 0.001 per min with BAEE as substrate at pH 7.6 at 25 °C; reaction volume 3.2 ml, 1 cm light path.
Preparation Note
Further purification of T9128 yielding an electrophoretically pure Kunitz inhibitor with increased activity.
Trypsin inhibitor is soluble in water and phosphate buffers at concentrations of 10 mg/ml or higher. Solutions at higher concentrations may be hazy and have a yellow to amber color.
Analysis Note
One mg will inhibit ≥1.0 mg of trypsin with activity of approx. 10,000 BAEE units per mg protein.
Other Notes
View more information on Trypsin Inhibitor.
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A midgut lysate of the Riptortus pedestris has antibacterial activity against LPS O-antigen-deficient Burkholderia mutants
Am Jang H, et al.
Developmental and Comparative Immunology, 67, 97-106 (2017)
Xingfei Li et al.
Journal of agricultural and food chemistry, 66(17), 4439-4448 (2018-03-23)
We first observed that protein/polysaccharide interaction exhibited noninteracting behavior which makes Bowman-Birk chymotrypsin inhibitor (BBI) always free of complexation, being separated from another protein with similar isoelectric points, Kunitz trypsin inhibitor (KTI). Turbidity titrations showed that the electrostatic attractions were
Functional analysis of the Kunitz trypsin inhibitor family in poplar reveals biochemical diversity and multiplicity in defense against herbivores
Major IT and Constabel CP
Plant Physiology, 146(3), 888-903 (2008)
A continuous fluorometric assay for trypsin based on melittin and the noncovalent-binding-induced pyrene excimer
Xu N, et al.
Chemistry Letters (Jpn), 42(12), 1528-1530 (2013)
Quantitative determination of active Bowman-Birk isoinhibitors, IBB1 and IBBD2, in commercial soymilks
Arques MC, et al.
Food Chemistry, 155, 24-30 (2014)
Protocols
This technical article described the Enzymatic Assay of Trypsin Inhibitor.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service