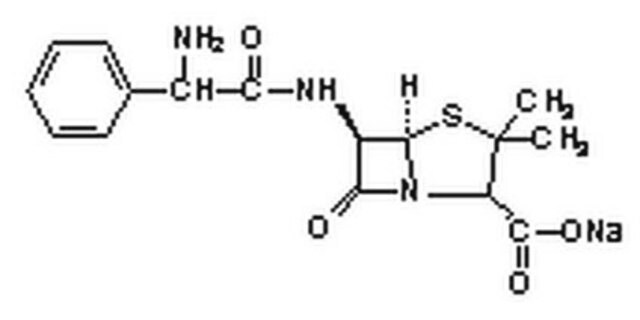
A0166
Ampicillin sodium salt
powder or crystals, BioReagent, suitable for cell culture
Synonym(s):
D-(−)-α-Aminobenzylpenicillin sodium salt
About This Item
Recommended Products
biological source
synthetic
Quality Level
product line
BioReagent
form
powder or crystals
potency
845-988 μg per mg (C16H18N3O4S, Calculated on the anhydrous basis)
technique(s)
cell culture | mammalian: suitable
color
white to off-white
mp
215 °C (dec.) (lit.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
[Na+].CC1(C)SC2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C([O-])=O
InChI
1S/C16H19N3O4S.Na/c1-16(2)11(15(22)23)19-13(21)10(14(19)24-16)18-12(20)9(17)8-6-4-3-5-7-8;/h3-7,9-11,14H,17H2,1-2H3,(H,18,20)(H,22,23);/q;+1/p-1/t9-,10-,11+,14-;/m1./s1
InChI key
KLOHDWPABZXLGI-YWUHCJSESA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
In research, ampicillin plays a pivotal role in microbiological, biochemical, and cell culture investigations. Its utilization in laboratories extends to studying antibiotic resistance and penetration limitations, exploring the synergistic interactions between multiple antibiotics, and serving as a crucial component for the selection and maintenance of recombinant plasmids in E. coli. Through these applications, ampicillin sodium salt contributes significantly to advancing the understanding of antibiotic efficacy, bacterial responses, and molecular processes, making it an indispensable tool in various facets of scientific research.
Application
Recommended for antibacterial use in cell culture media at 100 mg/L.
Recommended for use in ampicillin-resistance studies at 20-125 μg/ml.
Biochem/physiol Actions
Mode of Resistance: Administration with ß-lactamase cleaves the ß-lactam ring of Ampicillin and inactivates it.
Antimicrobial Spectrum: Includes both gram-positive (similar to benzylpenicillin) and gram-negative bacteria (similar to tetracyclines and chloramphenicol.
Features and Benefits
- High quality antibiotic suitable for multiple research applications
- Broad-spectrum antibiotic
- Inhibits bacterial cell-wall synthesis
- Active against Gram-positive and Gram-negative bacteria
- Commonly used in Cell Culture, Cell Biology and Biochemical research
Packaging
Caution
Preparation Note
Storage and Stability
Other Notes
comparable product
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1A - Skin Sens. 1A
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
USP HPLC Analysis of Ampicillin Sodium on Ascentis® Express C18
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service