Recommended Products
biological source
rabbit muscle
Quality Level
form
suspension
specific activity
~550 units/mg protein (at 25 °C (1,100 U/mg at 37 °C) with pyruvate as the substrate.)
mol wt
140,000 Da
packaging
pkg of 10 mL (10127884001 [100 mg])
pkg of 2 mL (10127230001 [10 mg])
pkg of 5 mL (10127876001 [25 mg])
manufacturer/tradename
Roche
technique(s)
activity assay: suitable
color
white
pH
6.0-7.0
solubility
water: miscible
NCBI accession no.
UniProt accession no.
application(s)
life science and biopharma
foreign activity
Aldolase <0.001%
GOT <0.01%
GPT <0.01%
MDH <0.01%
PK <0.001%
myokinase <0.01%
shipped in
wet ice
storage temp.
2-8°C
Gene Information
rabbit ... LOC100355262(100355262)
Related Categories
General description
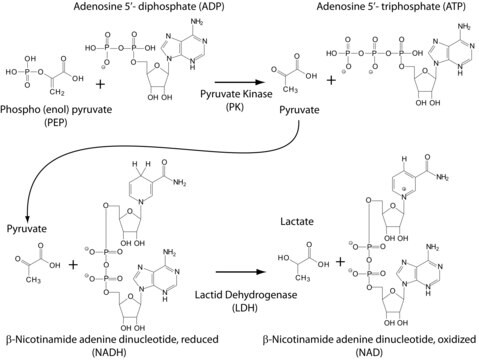
L-lactate dehydrogenase catalyzes the reversible reduction of pyruvate to L-lactate.
Specificity
Application
Sequence
Therefore, 5 electrophoretically distinguishable LDH isoenzymes, LDH-1 through LDH-5, of differing subunit composition, are found in mammals.
The molecular weight of all LDH isoenzymes is approximately the same.
– LDH-1 (H4), LDH-2 (H3M): predominant components of heart LDH
– LDH-3 (H2M2): principal component of LDH from lymphatic tissue
– LDH-4 (HM3), LDH-5 (M4): predominate in skeletal muscle or liver LDH
Note: The M subunit of LDH used to be designated the A subunit; the H subunit was the B subunit. Under the old designation, LDH-1 isoenzyme was LDH-B4.
Unit Definition
Note: For preparations H and I, the activity is defined at +30 °C, pH 7.8. The above assay consumes 1 mol of NADH per mol of pyruvate reduced.
Unit Conversion: For the reduction reaction, pyruvate as substrate:
1 U (+25 °C) 1.5 U (+37 °C) [hog muscle LDH in glycerol].
1 U (+25 °C) 1.8 U (+37 °C) [hog muscle LDH in amm onium sulfate].
1 U (+25 °C) 1.1 U (+30 °C) 2.0 U (+37 °C) [rabbit muscle LDH].
1 U (+25 °C) 1.4 U(+30 °C) 2.5 U (+37 °C) [pig heart LDH].
1 U (+25 °C) 2.5 U (+37 °C) [beef heart LDH].
Physical form
Preparation Note
Analysis Note
Other Notes
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service